利用电化学原位生成的 BH3 进行 CBS 催化的芳基甲基酮不对称还原研究
IF 3.5
4区 化学
Q2 ELECTROCHEMISTRY
引用次数: 0
摘要
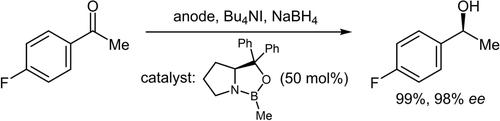
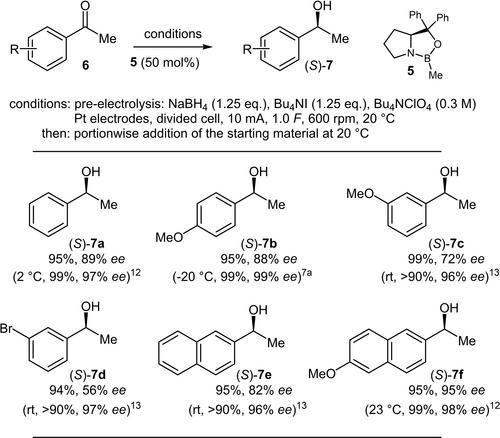
这项研究的目的是探索利用 CBS 型催化剂在电化学条件下对原手性芳基甲基酮进行不对称还原的可能性,以便在 NaBH4 与阳极室中原位生成的 I2 氧化后生成所需的 BH3。因此,我们对各种电化学参数进行了优化,以便在高产率和高立体化学诱导下形成所需的手性仲醇,尽管催化剂的负载量必须选择得相对较高,以便与电生成的 BH3 对酮的外消旋还原相一致。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Investigation Towards the Asymmetric CBS-Catalysed Reduction of Aryl Methyl Ketones with Electrochemically in Situ Generated BH3
The aim of this investigation was to explore the possibility to perform an asymmetric reduction, utilising a CBS-type catalyst, of prochiral aryl methyl ketones under electrochemical conditions to generate the needed BH3 upon oxidation of NaBH4 with in situ generated I2 in the anode compartment. Therefore, various electrochemical parameters were optimised to conduct the desired formation of the chiral secondary alcohols in high to quantitative yields with a high stereochemical induction, although the catalyst loading had to be chosen relatively high to concur with the racemic reduction of the ketones by the electrogenerated BH3.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ChemElectroChem
ELECTROCHEMISTRY-
CiteScore
7.90
自引率
2.50%
发文量
515
审稿时长
1.2 months
期刊介绍:
ChemElectroChem is aimed to become a top-ranking electrochemistry journal for primary research papers and critical secondary information from authors across the world. The journal covers the entire scope of pure and applied electrochemistry, the latter encompassing (among others) energy applications, electrochemistry at interfaces (including surfaces), photoelectrochemistry and bioelectrochemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


