cGAS 激活的内皮细胞-T 细胞交叉对话启动了三级淋巴结构的形成。
IF 17.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
环鸟苷单磷酸-腺苷单磷酸合成酶-干扰素基因刺激器(cGAS-STING)通路的异常激活会导致人类和小鼠的自身免疫病;然而,cGAS-STING通路启动适应性免疫和组织病理学的确切机制仍未完全明了。在这里,我们使用了一种会产生系统性自身免疫的 cGAS 基因敲除(KI)小鼠模型。在 cGAS-KI 小鼠的肺部,血管被类似三级淋巴结构(TLS)的有组织淋巴组织包围。细胞内cGAS诱导促进了CD8+ T细胞中CCR5的上调,并导致血管内皮细胞产生CCL5。外周 CD8+ T 细胞被招募到肺部,并产生 CXCL13 和干扰素-γ。后者会引发内皮细胞死亡,促进 CCL5 的产生,并且对 TLS 的建立至关重要。阻断 CCL5 或 CCR5 或消耗 CD8+ T 细胞都会阻碍 TLS 的形成。这些数据表明,cGAS 信号传导驱动着一种特化的淋巴结构,而这种淋巴结构是自身免疫组织病理学的基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
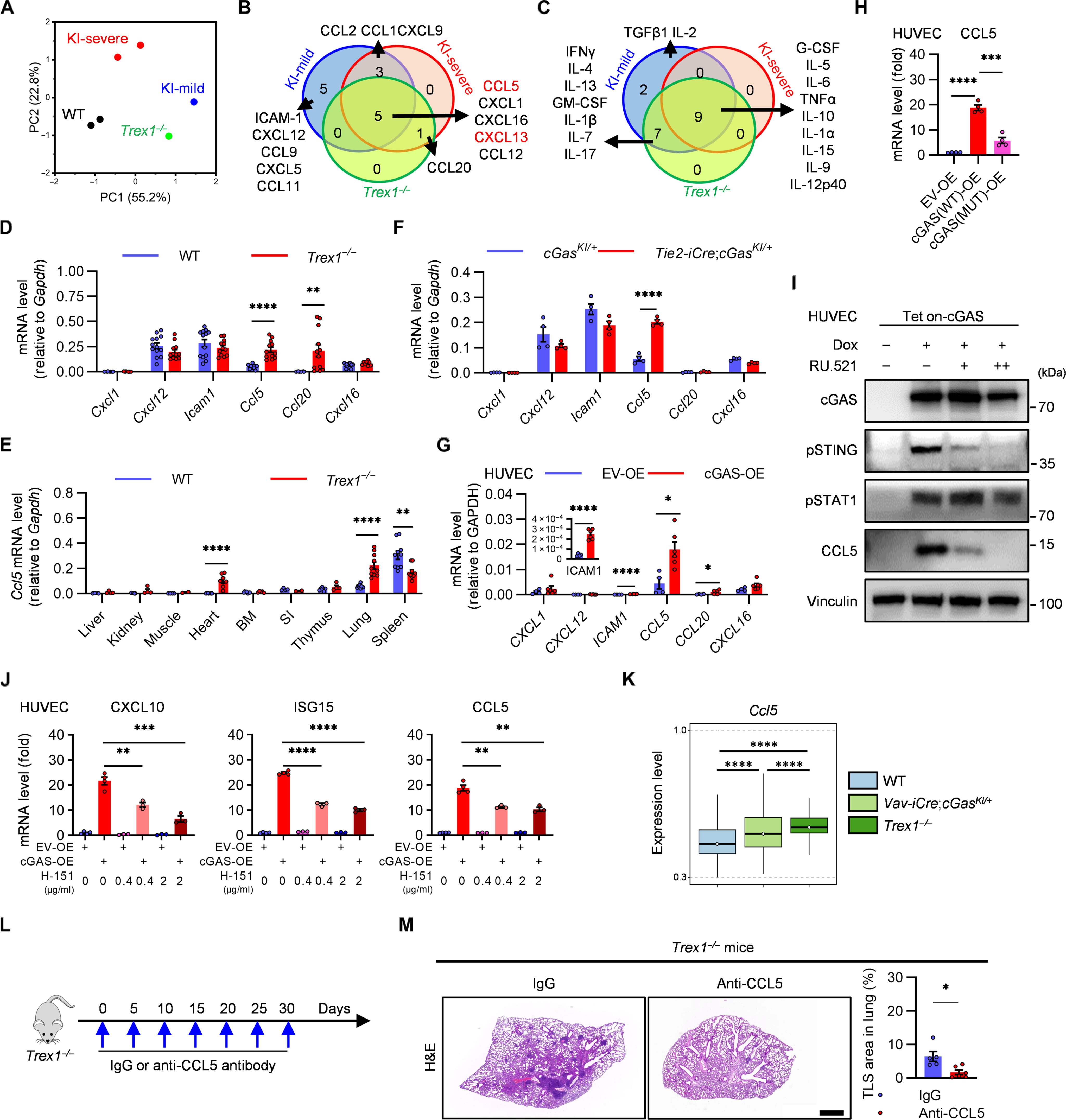
cGAS-activated endothelial cell–T cell cross-talk initiates tertiary lymphoid structure formation
Aberrant activation of the cyclic guanosine monophosphate–adenosine monophosphate synthase–stimulator of interferon genes (cGAS-STING) pathway causes autoimmunity in humans and mice; however, the exact mechanism by which the cGAS-STING pathway initiates adaptive immunity and tissue pathology is still not fully understood. Here, we used a cGAS knockin (KI) mouse model that develops systemic autoimmunity. In the lungs of cGAS-KI mice, blood vessels were enclosed by organized lymphoid tissues that resemble tertiary lymphoid structures (TLSs). Cell-intrinsic cGAS induction promoted up-regulation of CCR5 in CD8+ T cells and led to CCL5 production in vascular endothelial cells. Peripheral CD8+ T cells were recruited to the lungs and produced CXCL13 and interferon-γ. The latter triggered endothelial cell death, potentiated CCL5 production, and was essential for TLS establishment. Blocking CCL5 or CCR5, or depleting CD8+ T cells, impaired TLS formation. cGAS-mediated TLS formation also enhanced humoral and antitumor responses. These data demonstrate that cGAS signaling drives a specialized lymphoid structure that underlies autoimmune tissue pathology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Immunology
Immunology and Microbiology-Immunology
CiteScore
32.90
自引率
2.00%
发文量
183
期刊介绍:
Science Immunology is a peer-reviewed journal that publishes original research articles in the field of immunology. The journal encourages the submission of research findings from all areas of immunology, including studies on innate and adaptive immunity, immune cell development and differentiation, immunogenomics, systems immunology, structural immunology, antigen presentation, immunometabolism, and mucosal immunology. Additionally, the journal covers research on immune contributions to health and disease, such as host defense, inflammation, cancer immunology, autoimmunity, allergy, transplantation, and immunodeficiency. Science Immunology maintains the same high-quality standard as other journals in the Science family and aims to facilitate understanding of the immune system by showcasing innovative advances in immunology research from all organisms and model systems, including humans.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


