基于脂质和聚合物的体内免疫细胞基因递送纳米粒子靶向策略
IF 8.3
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
脂质纳米粒子和聚合物纳米粒子是一种前景广阔的生物材料平台,可用于细胞内DNA和mRNA的强效递送,基于纳米粒子(NP)的mRNA疫苗的广泛应用有助于结束COVID-19大流行。最近的研究试图将这种纳米技术应用于体内免疫细胞的转染和改造。免疫系统是一个特别有吸引力的目标,因为它参与了许多不同疾病的治疗,体内工程免疫细胞疗法,如嵌合抗原受体(CAR)T疗法,已经在某些血癌的临床治疗中取得了显著的成功。虽然基因递送有可能解决目前自体免疫细胞疗法在成本和生产方面的一些问题,但在体内转染免疫细胞仍具有挑战性。不仅很难将肝外 NP 运送到淋巴器官,而且 T 细胞等免疫细胞对转染也表现出特别的抵抗力。尽管存在这些挑战,NPs 的模块化特性使研究人员能够研究颗粒特性与其在体内特异性设计免疫细胞的能力之间的关键结构-功能关系。本文概述了几种纳米材料成分,包括靶向配体、核酸载体、化学特性、物理特性和给药途径,以便将 NPs 特异性地靶向免疫细胞,实现最佳体内转染。本文章由计算机程序翻译,如有差异,请以英文原文为准。
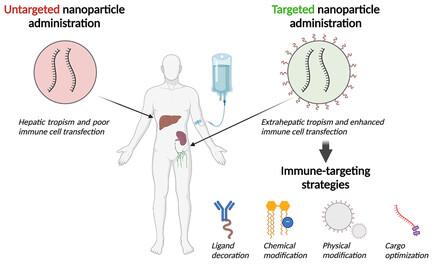
Nanoparticle Targeting Strategies for Lipid and Polymer-Based Gene Delivery to Immune Cells In Vivo
Lipid nanoparticles and polymeric nanoparticles are promising biomaterial platforms for robust intracellular DNA and mRNA delivery, highlighted by the widespread use of nanoparticle- (NP) based mRNA vaccines to help end the COVID-19 pandemic. Recent research has sought to adapt this nanotechnology to transfect and engineer immune cells in vivo. The immune system is an especially appealing target due to its involvement in many different diseases, and ex vivo-engineered immune cell therapies like chimeric antigen receptor (CAR) T therapy have already demonstrated remarkable clinical success in certain blood cancers. Although gene delivery can potentially address some of the cost and manufacturing concerns associated with current autologous immune cell therapies, transfecting immune cells in vivo is challenging. Not only is extrahepatic NP delivery to lymphoid organs difficult, but immune cells like T cells have demonstrated particular resistance to transfection. Despite these challenges, the modular nature of NPs allows researchers to examine critical structure–function relationships between a particle's properties and its ability to specifically engineer immune cells in vivo. Herein, several nanomaterial components are outlined, including targeting ligands, nucleic acid cargo, chemical properties, physical properties, and the route of administration to specifically target NPs to immune cells for optimal in vivo transfection.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
14.00
自引率
2.40%
发文量
0
期刊介绍:
Small Science is a premium multidisciplinary open access journal dedicated to publishing impactful research from all areas of nanoscience and nanotechnology. It features interdisciplinary original research and focused review articles on relevant topics. The journal covers design, characterization, mechanism, technology, and application of micro-/nanoscale structures and systems in various fields including physics, chemistry, materials science, engineering, environmental science, life science, biology, and medicine. It welcomes innovative interdisciplinary research and its readership includes professionals from academia and industry in fields such as chemistry, physics, materials science, biology, engineering, and environmental and analytical science. Small Science is indexed and abstracted in CAS, DOAJ, Clarivate Analytics, ProQuest Central, Publicly Available Content Database, Science Database, SCOPUS, and Web of Science.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


