PIP5K1C 和 PIKfyve 的双重抑制剂可阻止 SARS-CoV-2 进入细胞。
IF 12.9
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
SARS-CoV-2 大流行对全球公共卫生和经济造成了前所未有的影响。尽管疫苗和抗病毒药物提供了有效的保护和治疗,但要改善 SARS-CoV-2 的临床治疗效果,开发新的小分子抗病毒候选药物势在必行。在这项研究中,我们发现 UNI418(一种 PIKfyve 和 PIP5K1C 双重抑制剂)是一种能抑制 SARS-CoV-2 进入宿主细胞的新型化学制剂。UNI418 可抑制由 PIKfyve 调节的蛋白酶的蛋白水解活化,从而抑制依赖于蛋白酶 L 的蛋白水解作用,将 SARS-CoV-2 穗状病毒蛋白裂解为成熟形式,这是病毒内体逃逸的关键步骤。我们还证明,UNI418 通过抑制 PIP5K1C 阻止了 ACE2 介导的病毒内吞。我们的研究结果将 PIKfyve 和 PIP5K1C 确定为潜在的抗病毒靶点,并将 UNI418 确定为一种可治疗 SARS-CoV-2 的化合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
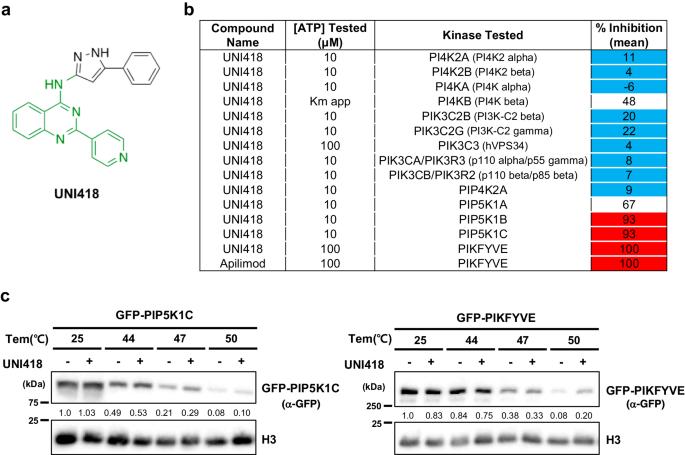
A dual inhibitor of PIP5K1C and PIKfyve prevents SARS-CoV-2 entry into cells
The SARS-CoV-2 pandemic has had an unprecedented impact on global public health and the economy. Although vaccines and antivirals have provided effective protection and treatment, the development of new small molecule-based antiviral candidates is imperative to improve clinical outcomes against SARS-CoV-2. In this study, we identified UNI418, a dual PIKfyve and PIP5K1C inhibitor, as a new chemical agent that inhibits SARS-CoV-2 entry into host cells. UNI418 inhibited the proteolytic activation of cathepsins, which is regulated by PIKfyve, resulting in the inhibition of cathepsin L-dependent proteolytic cleavage of the SARS-CoV-2 spike protein into its mature form, a critical step for viral endosomal escape. We also demonstrated that UNI418 prevented ACE2-mediated endocytosis of the virus via PIP5K1C inhibition. Our results identified PIKfyve and PIP5K1C as potential antiviral targets and UNI418 as a putative therapeutic compound against SARS-CoV-2. The COVID-19 pandemic, triggered by the SARS-CoV-2 virus, underscores the immediate need for effective treatments, particularly for severe cases. Even with vaccines, treatments that block the virus’s entry into cells are vital. SARS-CoV-2 enters host cells by attaching to the ACE2 receptor, a process that is a prime target for intervention. This research concentrates on blocking the virus’s entry into cells as a potential treatment method. The study is an experiment using cellular models to assess the effectiveness of a new compound, UNI418, in preventing SARS-CoV-2 infection. UNI418 targets enzymes involved in cell membrane dynamics, essential for the virus’s entry. The researchers conclude that UNI418, by blocking PIP5K1C and PIKfyve, offers a promising approach to preventing SARS-CoV-2 infection and emphasizes the importance of targeting the virus’s entry process as a treatment strategy. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


