中性粒细胞胞外捕获器介导斑块微环境与不稳定颈动脉斑块形成之间的相互影响。
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
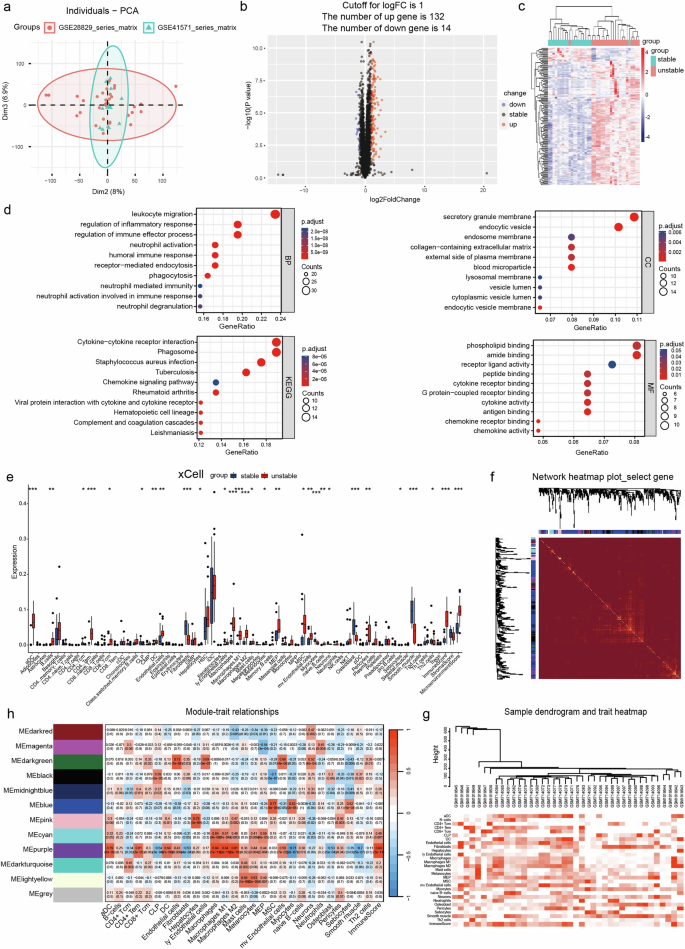
不稳定颈动脉粥样硬化斑块的形成与中性粒细胞胞外捕获物(NET)通过激活循环血液中的多种炎症介质诱导有关。然而,NETs 影响动脉粥样硬化斑块的微环境并导致不稳定颈动脉斑块发展的潜在机制在很大程度上仍然难以捉摸。本研究的目的是阐明髓系分化蛋白1(MD-1,LY86)诱导的NET在不稳定斑块形成和斑块微环境之间的相互作用。我们采用生物信息学分析方法确定了与颈动脉不稳定斑块相关的关键基因,然后利用各种实验方法对根据病理特征分类的组织标本和血浆样本进行了全面验证。颈动脉不稳定斑块患者的血浆中MD-1(LY86)浓度升高,而稳定斑块患者的浓度相对较低。此外,研究还发现可溶性 MD-1 可通过激活 Toll 样受体信号通路诱导 NET 的形成。NETs对内皮细胞的增殖和未成熟血管形成作用以及对细胞迁移的抑制作用与NETs的浓度直接相关。此外,研究还发现 NETs 能激活 NF-κB 信号通路,从而上调 ICAM1、VCAM1、MMP14、VEGFA 和 IL6 在人脐静脉内皮细胞(HUVECs)和 HAECs 中的表达。随后,NET 导致斑块内新生血管显著增加,导致颈动脉斑块稳定性变差,NET 反过来又刺激巨噬细胞产生更多的 MD-1,从而产生有害的正反馈循环。我们的研究结果表明,血液中的可溶性MD-1通过激活Toll样受体信号通路触发NET的产生,并进一步表明NET介导了颈动脉斑块微环境与斑块内新生血管之间的串联。抑制NETs的形成或MD-1的分泌可能是有效抑制不稳定颈动脉斑块发展的一种有前途的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Neutrophil extracellular traps mediate the crosstalk between plaque microenvironment and unstable carotid plaque formation
The development of unstable carotid atherosclerotic plaques is associated with the induction of neutrophil extracellular traps (NETs) via the activation of diverse inflammatory mediators in the circulating bloodstream. However, the underlying mechanisms through which NETs influence the microenvironment of atherosclerotic plaques and contribute to the development of unstable carotid plaques remain largely elusive. The objective of this study was to elucidate the role of myeloid differentiation protein 1 (MD-1, LY86)-induced NETs underlying the crosstalk between unstable plaque formation and the plaque microenvironment. We employed bioinformatics analysis to identify key genes associated with carotid-unstable plaque, followed by comprehensive validation using various experimental approaches on tissue specimens and plasma samples classified based on pathological characteristics. Patients with carotid-unstable plaques exhibited elevated plasma concentrations of MD-1 (LY86), while patients with stable plaques demonstrated comparatively lower levels. Furthermore, soluble MD-1 was found to induce the formation of NETs through activation of Toll-like receptor signaling pathway. The proliferative and immature vascularization effects of NETs on endothelial cells, as well as their inhibitory impact on cell migration, are directly correlated with the concentration of NETs. Additionally, NETs were found to activate the NF-κB signaling pathway, thereby upregulating ICAM1, VCAM1, MMP14, VEGFA, and IL6 expression in both Human umbilical vein endothelial cells (HUVECs) and HAECs. Subsequently, a significant increase in intraplaque neovascularization by NETs results in poor carotid plaque stability, and NETs in turn stimulate macrophages to produce more MD-1, generating a harmful positive feedback loop. Our findings suggest that soluble MD-1 in the bloodstream triggers the production of NETs through activation of the Toll-like receptor signaling pathway and further indicate NETs mediate a crosstalk between the microenvironment of the carotid plaque and the neovascularization of the intraplaque region. Inhibiting NETs formation or MD-1 secretion may represent a promising strategy to effectively suppress the development of unstable carotid plaques. Atherosclerosis, a disease where arteries get blocked with fat, is a main cause of heart disease and stroke. Predicting which atherosclerotic plaques will cause heart attacks is hard. Researchers analyzed gene data from unstable and stable carotid plaques, focusing on neutrophils and a protein called MD-1. The study involved 30 patients and 10 healthy volunteers to understand how MD-1 and neutrophils contribute to plaque instability. The main finding is that MD-1 could be a biomarker for unstable plaques, offering a new target for therapies to prevent major heart events. This progress in understanding the molecular mechanisms behind plaque instability could lead to better prevention strategies for heart disease. Future research may focus on developing treatments that target MD-1 and neutrophils to stabilize plaques and reduce the risk of heart attacks. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


