TFE3重排肾细胞癌的综合分子特征。
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
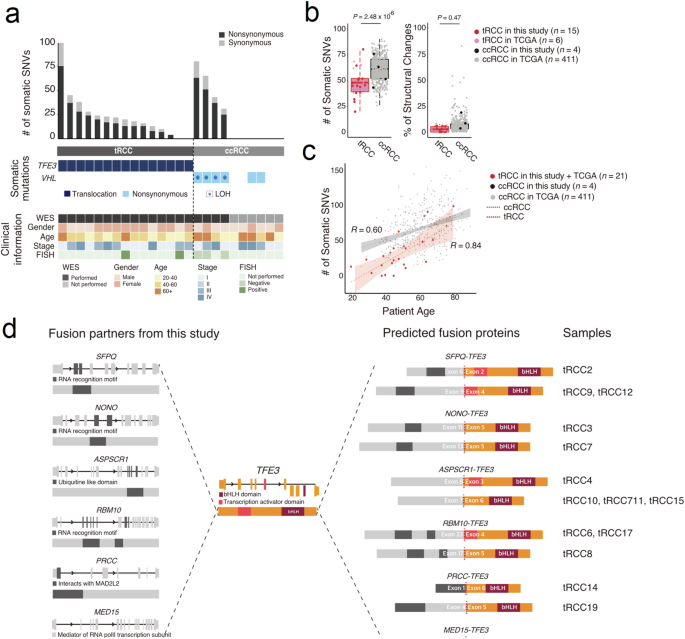
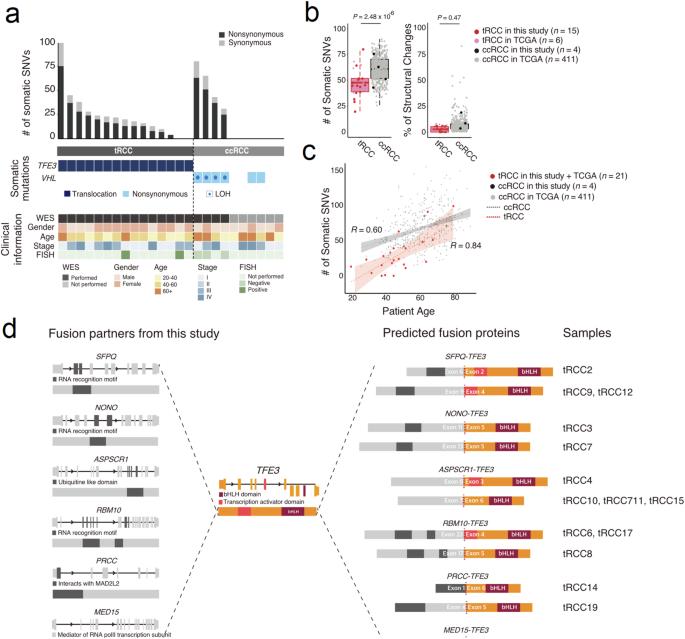
TFE3重排肾细胞癌(tRCC)是一种罕见的肾细胞癌,涉及Xp11.2 TFE3基因的染色体易位。尽管tRCC发病早、预后差,但其发病的分子机制仍然难以捉摸。本研究旨在为原发性和复发性 tRCC 患者确定新的治疗靶点。我们收集了19个经免疫组化确诊的TFE3阳性RCC组织,并对它们进行了基因鉴定,以检查它们的基因组和转录组特征。利用全外显子组测序(WES)和RNA测序(RNA-seq)数据提取了肿瘤特异性特征,并在TFE3易位的细胞系中分析了其功能性后果。结果发现,体细胞单核苷酸变异(SNV)的负担较低,体细胞变异的数量与发病年龄呈正相关。转录组分析表明,有四个样本(21.1%)缺乏预期的融合事件,并与透明细胞RCC(ccRCC)组织的基因组图谱聚集在一起。与ccRCC的表达图谱相比,融合事件还显示出与线粒体呼吸相关的基因富集上调。RNA 表达谱与 TFE3 ChIP-seq 模式数据的比较表明,PPARGC1A 是致癌过程的代谢调节因子。当 PPARGC1A 及其相关代谢通路被其抑制剂 SR-18292 抑制时,细胞增殖会减少。总之,我们证明 PPARGC1A 介导的线粒体呼吸可被视为 tRCC 的潜在治疗靶点。这项研究确定了一种具有独特临床特征的 RCC 亚型的未定性遗传特征,并提供了针对 tRCC 的治疗方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Comprehensive molecular characterization of TFE3-rearranged renal cell carcinoma
TFE3-rearranged renal cell cancer (tRCC) is a rare form of RCC that involves chromosomal translocation of the Xp11.2 TFE3 gene. Despite its early onset and poor prognosis, the molecular mechanisms of the pathogenesis of tRCC remain elusive. This study aimed to identify novel therapeutic targets for patients with primary and recurrent tRCC. We collected 19 TFE3-positive RCC tissues that were diagnosed by immunohistochemistry and subjected them to genetic characterization to examine their genomic and transcriptomic features. Tumor-specific signatures were extracted using whole exome sequencing (WES) and RNA sequencing (RNA-seq) data, and the functional consequences were analyzed in a cell line with TFE3 translocation. Both a low burden of somatic single nucleotide variants (SNVs) and a positive correlation between the number of somatic variants and age of onset were observed. Transcriptome analysis revealed that four samples (21.1%) lacked the expected fusion event and clustered with the genomic profiles of clear cell RCC (ccRCC) tissues. The fusion event also demonstrated an enrichment of upregulated genes associated with mitochondrial respiration compared with ccRCC expression profiles. Comparison of the RNA expression profile with the TFE3 ChIP-seq pattern data indicated that PPARGC1A is a metabolic regulator of the oncogenic process. Cell proliferation was reduced when PPARGC1A and its related metabolic pathways were repressed by its inhibitor SR-18292. In conclusion, we demonstrate that PPARGC1A-mediated mitochondrial respiration can be considered a potential therapeutic target in tRCC. This study identifies an uncharacterized genetic profile of an RCC subtype with unique clinical features and provides therapeutic options specific to tRCC. Understanding the unique traits of a rare kidney cancer type, TFE3-rearranged renal cell carcinoma, is important due to its poor response to usual treatments. This study explores the genetic and metabolic makeup of tRCC, comparing it with clear cell RCC and normal kidney cells. Using a mix of cell culture, whole exome sequencing, and various molecular analyses, the team conducted an experiment to reveal the unique genetic and metabolic profiles of tRCC. The researchers conclude that targeting the metabolic changes in tRCC, specifically through inhibiting PPARGC1A-mediated mitochondrial respiration, offers a new treatment approach. This approach marks a significant step in understanding and potentially treating tRCC. The implications of this study could lead to more effective treatments for patients with this challenging cancer type, emphasizing the importance of metabolic pathways in cancer therapy. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


