BMS-817399 的特性、溶解性和吸湿性
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
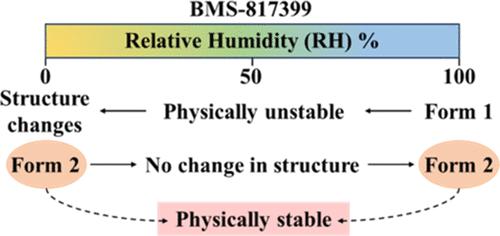
我们对 BMS-817399 的特性进行了研究,这些特性可能会影响该化合物作为活性药物成分 (API) 的潜在用途。该化合物以一水合物(形式 1)的形式存在,但由于其晶体结构会随着相对湿度和温度的变化而改变,因此在物理上并不稳定。因此,确定一种在相对湿度波动条件下保持物理稳定的形式是这项工作的目标。使用 Crystal16 仪器获得了形式 1 在乙醇、异丙醇、丙酮和乙腈中的溶解度,但只有在溶剂为乙腈时才会发生重结晶。在 Crystal16 仪器中重结晶得到的固体和更大规模实验得到的固体的 X 射线衍射(XRD)证实了该化合物新形式(形式 2)的存在。随后使用 Crystal16 仪器测定了形式 2 的溶解度。差示扫描量热法(DSC)和热重分析法(TGA)证实了形式 1 为一水合物,并表明形式 2 为无水物。动态水蒸气吸附试验表明,甲酸甲酯 1 的含水量随相对湿度的变化而变化。表 2 在相对湿度升高时也会吸湿,但 XRD 测量证实它具有物理稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Characterization, Solubility, and Hygroscopicity of BMS-817399
Characteristics of BMS-817399 that could influence the potential utility of the compound as an active pharmaceutical ingredient (API) have been explored. The compound exists as a monohydrate (Form 1) but is physically unstable as its crystal structure changes with variations in relative humidity and temperature. Thus, identifying a form that remains physically stable under fluctuating conditions of relative humidity was the objective of this work. Solubilities of Form 1 in ethanol, isopropanol, acetone, and acetonitrile were obtained using a Crystal16 apparatus, but recrystallization only occurred when the solvent was acetonitrile. X-ray diffraction (XRD) of solids obtained from recrystallization in the Crystal16 apparatus and from larger-scale experiments confirmed the existence of a new form (Form 2) of the compound. Solubilities of Form 2 were subsequently determined by using the Crystal16 apparatus. Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) confirmed Form 1 as a monohydrate and showed that Form 2 is anhydrous. Dynamic vapor sorption was used to show that the water content of Form 1 varied with the relative humidity. Form 2 also became hygroscopic at elevated relative humidity, but XRD measurements confirmed that it exhibited physical stability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


