2′-Deoxy-2′-fluoro-arabinoside 的全连续流合成:阿兹夫定的关键中间体
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
阿兹夫定于 2021 年在中国获批用于治疗成人 HIV-1 感染,并于 2022 年在中国获批有条件上市用于治疗 SARS-CoV-2 。在这项工作中,我们描述了阿兹夫定的关键中间体--2′-脱氧-2′-氟尿苷的全连续流合成过程。该过程在六个连续流装置中通过六次化学转化完成,包括氯化、水解、氟化、溴化、缩合和脱保护。在优化的工艺条件下,总停留时间为 156 分钟,总产率为 32.3%。与间歇条件相比,总产率提高了一倍,总反应时间缩短了 16 倍,E 系数降低了 1.63 倍。本文章由计算机程序翻译,如有差异,请以英文原文为准。
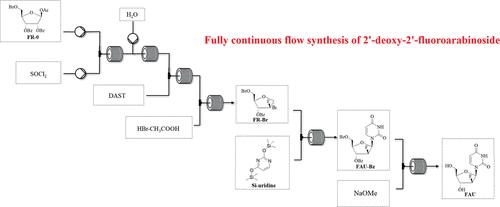
Fully Continuous Flow Synthesis of 2′-Deoxy-2′-fluoro-arabinoside: A Key Intermediate of Azvudine
Azvudine was approved for the treatment of adult HIV-1 infection in China in 2021, and it was approved for conditional marketing for the treatment of SARS-CoV-2 in China in 2022. In this work, we describe a fully continuous flow synthesis of 2′-deoxy-2′-fluoroarabinoside, a key intermediate for azvudine. The process was accomplished via six chemical transformations, including chlorination, hydrolysis, fluorination, bromination, condensation, and deprotection in six sequential continuous flow devices. Under the optimized process conditions, the total yield was 32.3% with a total residence time of 156 min. Compared with batch conditions, the total yield was doubled, the total reaction time was shortened 16 times, and the E factor was reduced 1.63 times.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


