原子分散过渡金属电催化剂的活性与稳定性
IF 79.8
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
以清洁和可持续的氢为燃料的聚合物电解质燃料电池是清洁交通的一种极具吸引力的解决方案。然而,聚合物电解质燃料电池成本高昂,因为需要使用大量的铂族金属催化剂,以催化阴极非常缓慢的氧还原反应。在这方面,最有吸引力的途径是用性能相似或更好的不含铂族金属的材料完全取代贵金属催化剂。自 2010 年以来,已经提出了许多有前途的无 PGM 电催化催化剂。然而,性能最好的催化剂还不能满足实际系统的要求。催化剂发现过程中的一个重要障碍是严重依赖经验而非基于理性的设计方法。本视角文章重点介绍基于原子分散的氮配位单原子金属位点(M-N-C 催化剂)的最有前途的无 PGM 氧还原反应催化剂。我们特别关注活性位点结构以及影响催化活性和性能耐久性的关键因素。我们提出了通过在原子尺度、中观尺度和纳米尺度上控制催化剂结构来提高性能的潜在有效策略。我们强调了克服经常观察到的活性-稳定性权衡的重要性,以及先进建模对催化剂合理设计的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
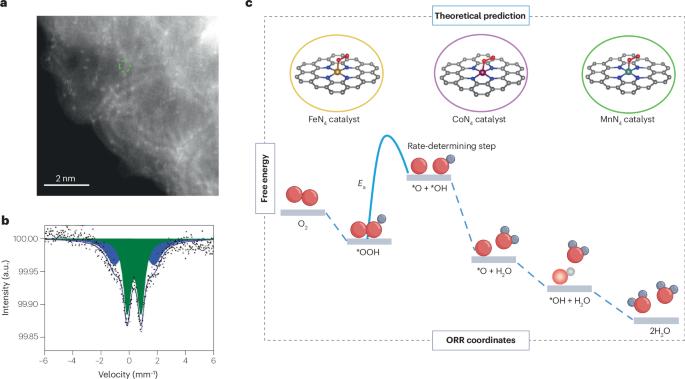
Activity versus stability of atomically dispersed transition-metal electrocatalysts
Polymer electrolyte fuel cells operating on clean and sustainable hydrogen are an attractive solution for clean transportation. However, polymer electrolyte fuel cells are costly owing to the use of considerable amounts of platinum group metal (PGM) catalysts, which are needed to catalyse the very slow oxygen reduction reaction at the cathode. The most attractive path in that regard is a complete replacement of precious metal catalysts by PGM-free materials with similar or better performance. Since 2010, numerous promising catalysts have been proposed for PGM-free electrocatalysis. However, the best-performing catalysts do not yet meet the requirements of practical systems. One important hurdle in catalyst discovery is relying heavily on empirical rather than rational design-based approaches. This Perspective article focuses on the most promising PGM-free oxygen reduction reaction catalysts based on atomically dispersed, nitrogen-coordinated single-atom metal sites (M–N–C catalysts). We specifically concentrate on the active-site structure and critical factors governing catalytic activity and performance durability. We propose potentially effective strategies for improving performance by controlling the catalyst structure at the atomic scale, mesoscale and nanoscale. We highlight the importance of overcoming often-observed activity–stability trade-offs and the importance of advanced modelling for the rational design of catalysts. Platinum group metal-free electrocatalysts that utilize atomically dispersed, nitrogen-coordinated transition-metal sites in carbon are a promising replacement for platinum-based oxygen reduction reaction catalysts in fuel cells. This Perspective article offers a concise discussion on addressing remaining challenges related to activity–stability trade-offs by precisely controlling catalyst structures at multiple scales.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Materials
Materials Science-Biomaterials
CiteScore
119.40
自引率
0.40%
发文量
107
期刊介绍:
Nature Reviews Materials is an online-only journal that is published weekly. It covers a wide range of scientific disciplines within materials science. The journal includes Reviews, Perspectives, and Comments.
Nature Reviews Materials focuses on various aspects of materials science, including the making, measuring, modelling, and manufacturing of materials. It examines the entire process of materials science, from laboratory discovery to the development of functional devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


