平衡腺苷 A2A 受体中 G 蛋白的选择性和功效
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
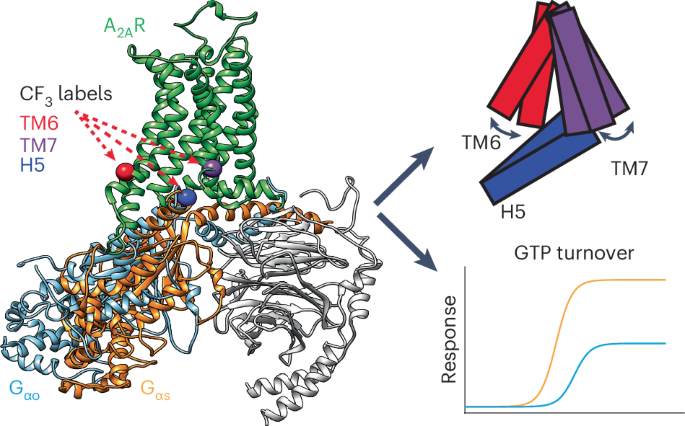
腺苷 A2A 受体(A2AR)与多种 G 蛋白(尤其是 Go 及其同源 Gs 蛋白)发生作用。A2AR 和 G 蛋白的 19F 核磁共振成像显示,动态组合促进了这种耦合杂交。以前与部分激动和完全激动相关的两种跨膜螺旋 6 (TM6) 激活状态适应了 Gs 和 Go 的不同体积。在 A2AR-Gs 中,核苷酸耗竭会使 TM7 偏向完全激活状态,而 A2AR-Go 则以动态非激活/中间部分为特征。分子动力学模拟显示,NPxxY 矩阵是一个高度保守的开关,它在 A2AR-Go 复合物中建立了一种独特的配置,不能稳定与 Gs 的螺旋-8 接口,并采用活性状态。由此产生的 TM7 动态阻碍了 G 蛋白的耦合,这表明动力学门控可能是非识别 G 蛋白复合物功效降低的原因。因此,双重 TM6 激活状态使耦合伙伴更加多样化,而 TM7 动态则决定了耦合效率。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Balancing G protein selectivity and efficacy in the adenosine A2A receptor
The adenosine A2A receptor (A2AR) engages several G proteins, notably Go and its cognate Gs protein. This coupling promiscuity is facilitated by a dynamic ensemble, revealed by 19F nuclear magnetic resonance imaging of A2AR and G protein. Two transmembrane helix 6 (TM6) activation states, formerly associated with partial and full agonism, accommodate the differing volumes of Gs and Go. While nucleotide depletion biases TM7 toward a fully active state in A2AR–Gs, A2AR–Go is characterized by a dynamic inactive/intermediate fraction. Molecular dynamics simulations reveal that the NPxxY motif, a highly conserved switch, establishes a unique configuration in the A2AR–Go complex, failing to stabilize the helix-8 interface with Gs, and adoption of the active state. The resulting TM7 dynamics hamper G protein coupling, suggesting kinetic gating may be responsible for reduced efficacy in the noncognate G protein complex. Thus, dual TM6 activation states enable greater diversity of coupling partners while TM7 dynamics dictate coupling efficacy. Fluorine-19 NMR analysis combined with molecular dynamics and Monte Carlo simulations captures the activation ensemble and associated dynamics of adenosine A2A receptor-mediated coupling to cognate and noncognate G proteins.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


