利用 BANC-seq 量化全基因组转录因子与染色质的结合亲和力。
IF 13.1
1区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
转录因子(TFs)结合特定的 DNA 序列来调控转录。除 DNA 序列外,DNA 可及性和染色质结构等局部因素也决定了 TF 与任何给定基因座的亲和力。事实证明,在测量 TF-DNA 亲和力时,很难将这些因素包括在内。为了应对这一挑战,我们最近开发了一种名为原生染色质结合亲和力测序(BANC-seq)的方法。在 BANC-seq 中,用一定浓度范围的表位标记 TF 培养完整的哺乳动物细胞核,然后进行染色质免疫沉淀或在靶标下裂解,再用核酸酶释放尖峰 DNA。这样就能确定表观解离常数(KdApp)值,该值由发生半最大结合时的 TF 浓度定义,涉及整个基因组。在此,我们介绍了 BANC-seq 的详细步骤方案,包括下游数据分析。原则上,任何分子生物学家都能在短短 1.5 天内完成 BANC-seq 实验(不包括分析)。不过,测序数据的预处理和分析需要一定的命令行 shell 和 R 编程经验。本文章由计算机程序翻译,如有差异,请以英文原文为准。

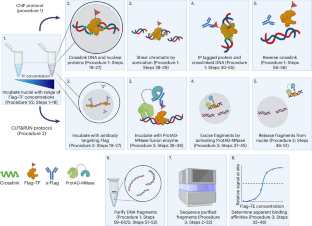
Quantifying genome-wide transcription factor binding affinities for chromatin using BANC-seq
Transcription factors (TFs) bind specific DNA sequences to regulate transcription. Apart from DNA sequences, local factors such as DNA accessibility and chromatin structure determine the affinity of a TF for any given locus. Including these factors when measuring TF–DNA affinities has proven difficult. To address this challenge, we recently developed a method called binding affinities in native chromatin by sequencing (BANC-seq). In BANC-seq, intact mammalian nuclei are incubated with a concentration range of epitope-tagged TF, followed by either chromatin immunoprecipitation or cleavage under target and release using nuclease with spike-in DNA. This allows determination of apparent dissociation constant (KdApp) values, defined by the concentration of TF at which half-maximum binding occurs, across the genome. Here we present a detailed stepwise protocol for BANC-seq, including downstream data analysis. In principle, any molecular biologist should be able to perform a BANC-seq experiment in as little as 1.5 d (excluding analysis). However, preprocessing and analysis of the sequencing data does require some experience in command-line shell and R programming. BANC-seq enables quantification of genome-wide transcription factor binding affinities in the native chromatin context. This protocol describes implementations based on chromatin immunoprecipitation or cleavage under target and release using nuclease, followed by library preparation, sequencing and data analysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Protocols
生物-生化研究方法
CiteScore
29.10
自引率
0.70%
发文量
128
审稿时长
4 months
期刊介绍:
Nature Protocols focuses on publishing protocols used to address significant biological and biomedical science research questions, including methods grounded in physics and chemistry with practical applications to biological problems. The journal caters to a primary audience of research scientists and, as such, exclusively publishes protocols with research applications. Protocols primarily aimed at influencing patient management and treatment decisions are not featured.
The specific techniques covered encompass a wide range, including but not limited to: Biochemistry, Cell biology, Cell culture, Chemical modification, Computational biology, Developmental biology, Epigenomics, Genetic analysis, Genetic modification, Genomics, Imaging, Immunology, Isolation, purification, and separation, Lipidomics, Metabolomics, Microbiology, Model organisms, Nanotechnology, Neuroscience, Nucleic-acid-based molecular biology, Pharmacology, Plant biology, Protein analysis, Proteomics, Spectroscopy, Structural biology, Synthetic chemistry, Tissue culture, Toxicology, and Virology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


