20(S)-人参皂苷 Rh2 通过抑制 Src/STAT3 信号传导诱导黑色素瘤细胞凋亡和自噬
IF 6.8
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
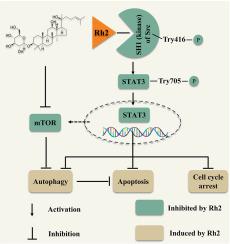
背景20(S)-人参皂苷Rh2(GRh2)具有多方面的健康益处,已被广泛研究。然而,人们对 GRh2 的抗黑色素瘤作用仍然知之甚少。方法采用MTT试验、EdU染色试验、流式细胞分析、细胞热转移试验(CETSA)、共聚焦显微镜分析、分子对接、分子动力学(MD)、免疫印迹、B16F10细胞小鼠模型等方法研究GRh2的抗黑色素瘤作用及其机制。结果 在黑色素瘤细胞中,GRh2能抑制细胞增殖,使细胞周期停滞在G0/G1期,并诱导细胞凋亡。GRh2 在黑色素瘤细胞中启动了自噬,并抑制了自噬负调控因子 mTOR 的活性。抑制自噬可增强 GRh2 的抗黑色素瘤功效。分子对接、MD 和 CETSA 研究显示,GRh2 与 Src 蛋白(STAT3 的上游激酶之一)稳定结合。GRh2 抑制了 Src 和 STAT3 的活性,从而禁止了 STAT3 在黑色素瘤细胞中的核转位。STAT3 的过度激活削弱了 GRh2 的细胞毒性、凋亡和自噬诱导作用。此外,GRh2 还能抑制 B16F10 肿瘤的生长,但不会对小鼠产生明显的毒性。结论GRh2通过抑制Src/STAT3信号转导发挥抗黑色素瘤作用。这项研究加深了我们对 GRh2 抗黑色素瘤机制的了解,并表明摄入 GRh2 有可能延缓黑色素瘤的进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
20(S)-Ginsenoside Rh2 induces apoptosis and autophagy in melanoma cells via suppressing Src/STAT3 signaling
Background
20(S)-Ginsenoside Rh2 (GRh2) has been extensively studied for multifaceted health benefits. However, the anti-melanoma effect of GRh2 remains poorly understood. Herein, the anti-melanoma effects and underlying mechanisms of GRh2 were investigated.
Methods
MTT assays, the EdU staining assay, flow cytometric analysis, the cellular thermal shift assay (CETSA), confocal microscope analysis, molecular docking, molecular dynamics (MD), immunoblotting, a B16F10 cell bearing mouse model were adopted to examine the anti-melanoma effect of mechanism of action of GRh2.
Results
In melanoma cells, GRh2 was found to suppress cell proliferation, arrest cell cycle at G0/G1 phase and evoke apoptosis. GRh2 initiated autophagy and inhibited the activity of mTOR, the autophagy negative regulator, in melanoma cells. Repressing autophagy enhanced the anti-melanoma efficacy of GRh2. Molecular docking, MD and CETSA studies revealed that GRh2 stably bound to Src protein (one of the upstream kinases of STAT3). GRh2 suppressed Src and STAT3 activities, thereof prohibiting STAT3 nuclear translocation in melanoma cells. STAT3 over-activation attenuated the cytotoxic, apoptotic and autophagy inductive effects of GRh2. Additionally, GRh2 suppressed B16F10 tumor growth without inducing obvious toxicity in mice. It downregulated phospho-Src, phospho-STAT3, phospho-mTOR and Mcl-1 protein levels, while elevated cleaved-PARP and LC3B-II protein levels in B16F10 tumors.
Conclusion
GRh2 exerts anti-melanoma effects through suppressing Src/STAT3 signaling. This study advances our understanding on the anti-melanoma mechanism of GRh2 and indicates that the intake of GRh2 has the potential to retard melanoma progression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Ginseng Research
CHEMISTRY, MEDICINAL-INTEGRATIVE & COMPLEMENTARY MEDICINE
CiteScore
11.40
自引率
9.50%
发文量
111
审稿时长
6-12 weeks
期刊介绍:
Journal of Ginseng Research (JGR) is an official, open access journal of the Korean Society of Ginseng and is the only international journal publishing scholarly reports on ginseng research in the world. The journal is a bimonthly peer-reviewed publication featuring high-quality studies related to basic, pre-clinical, and clinical researches on ginseng to reflect recent progresses in ginseng research.
JGR publishes papers, either experimental or theoretical, that advance our understanding of ginseng science, including plant sciences, biology, chemistry, pharmacology, toxicology, pharmacokinetics, veterinary medicine, biochemistry, manufacture, and clinical study of ginseng since 1976. It also includes the new paradigm of integrative research, covering alternative medicinal approaches. Article types considered for publication include review articles, original research articles, and brief reports.
JGR helps researchers to understand mechanisms for traditional efficacy of ginseng and to put their clinical evidence together. It provides balanced information on basic science and clinical applications to researchers, manufacturers, practitioners, teachers, scholars, and medical doctors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


