糖源烯丙基醇的非对映选择性 1,3-双极分子内硝酮烯烃环加成 (INOC) 反应:功能化氨基环戊醇的合成。
IF 2.4
3区 化学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
从 3-O-benzyll-1,2-isopropylidene-α-D-xylo-pentodialdo-1,4-furanose 中得到的 Baylis-Hillman 糖加合物的 DIBAL-H 还原反应通过消除 β- 羟基得到了三取代烯。随后,将异亚丙基缩醛水解为相应的半缩醛,并与 N-苄基羟胺盐酸盐反应生成腈酮,腈酮经过非对映选择性分子内 1,3 双极腈酮烯烃环加成(INOC)反应生成异恶唑烷骨架。同时对融合异噁唑烷的 N-O 键和苄基进行还原裂解,以良好的产率获得了新的功能化氨基环戊醇。本文章由计算机程序翻译,如有差异,请以英文原文为准。
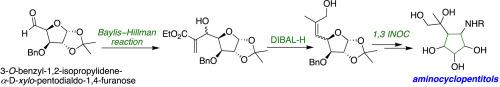
Diastereoselective 1,3-dipolar intramolecular nitrone olefin cycloaddition (INOC) reaction of a sugar-derived allyl alcohol: Synthesis of functionalized aminocyclopentitols
The DIBAL-H reduction of the Baylis-Hillman sugar adduct, obtained from 3-O-benzyl-1,2-isopropylidene-α-D-xylo-pentodialdo-1,4-furanose yielded trisubstituted alkenes by eliminating the β-hydroxyl group. Subsequently, the hydrolysis of the isopropylidene acetal to the corresponding hemiacetal was reacted with N-benzyl hydroxylamine hydrochloride to generate the nitrone, which underwent diastereoselective intramolecular 1,3-dipolar nitrone olefin cycloaddition (INOC) to give an isoxazolidine skeleton. The concomitant reductive cleavage of the N-O bond and benzyl group of the fused isoxazolidines afforded new functionalized aminocyclopentitols in good yields.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Carbohydrate Research
化学-生化与分子生物学
CiteScore
5.00
自引率
3.20%
发文量
183
审稿时长
3.6 weeks
期刊介绍:
Carbohydrate Research publishes reports of original research in the following areas of carbohydrate science: action of enzymes, analytical chemistry, biochemistry (biosynthesis, degradation, structural and functional biochemistry, conformation, molecular recognition, enzyme mechanisms, carbohydrate-processing enzymes, including glycosidases and glycosyltransferases), chemical synthesis, isolation of natural products, physicochemical studies, reactions and their mechanisms, the study of structures and stereochemistry, and technological aspects.
Papers on polysaccharides should have a "molecular" component; that is a paper on new or modified polysaccharides should include structural information and characterization in addition to the usual studies of rheological properties and the like. A paper on a new, naturally occurring polysaccharide should include structural information, defining monosaccharide components and linkage sequence.
Papers devoted wholly or partly to X-ray crystallographic studies, or to computational aspects (molecular mechanics or molecular orbital calculations, simulations via molecular dynamics), will be considered if they meet certain criteria. For computational papers the requirements are that the methods used be specified in sufficient detail to permit replication of the results, and that the conclusions be shown to have relevance to experimental observations - the authors'' own data or data from the literature. Specific directions for the presentation of X-ray data are given below under Results and "discussion".
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


