钯催化喹唑啉酮与芳香族羧酸的区域选择性交叉脱氢偶联
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
利用钯催化剂在 CH 活化作用下,开发了一种 2-芳基喹唑啉酮与不同苯基羧酸进行区域选择性交叉脱氢反应的简单方案。苯羧酸以简单易得的芳香族羧酸为来源。这种一锅法具有极高的区域选择性,并能以中等至极好的收率提供单苯并氧羰基化产物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
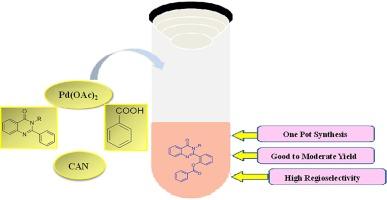
Pd-catalysed regioselective cross dehydrogenative coupling of quinazolinones with aromatic carboxylic acids
A simple protocol for a regioselective cross dehydrogenative reaction of 2-aryl-quinazolinones with different phenyl carboxylic acids was developed using a palladium catalyst facilitated by CH activation. Simple and readily accessible aromatic carboxylic acids were utilized as a source of benzoxylate. A one-pot method proceeded with great regioselectivity and offered mono benzoxylation product with moderate to excellent yield.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


