可见光诱导的未活化烯烃的三氟甲基化/环化:合成 CF3 取代的融合三环吲哚
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
据报道,通过可见光诱导的自由基三氟甲基化/未活化烯烃的环化反应,可以用容易获得的三氟甲基三噻吩鎓构建三氟甲基化的三环吲哚。在不添加光催化剂或添加剂的情况下,在室温下合成了多种含 CF3 的三环吲哚衍生物。机理研究表明,该反应可能是通过自由基途径进行的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
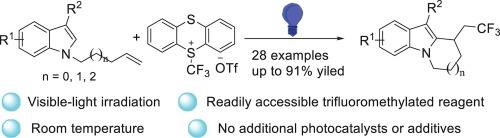
Visible-light-induced trifluoromethylation/cyclization of unactivated alkenes: Synthesis of CF3-substituted fused tricyclic indoles
A visible-light-induced radical trifluoromethylation/cyclization of unactivated alkenes for the construction of trifluoromethylated tricyclic indoles with readily accessible trifluoromethyl thianthrenium triflate has been reported. A variety of CF3-containing tricyclic indole derivatives were synthesized at room temperature without the addition of photocatalysts or additives. Mechanistic investigations indicate that the reaction probably undergo a radical pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


