通过构建分子异核配合物直接确定 CeIII-MnII 系统的能量传递机制
IF 9.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
金属中心禁止跃迁的敏化具有重要意义。具有 d-f 允许跃迁的 CeIII 对具有 d-d 禁止跃迁的 MnII 固体荧光粉的敏化是一种很有前景的光转换材料,但由于金属中心间距离的不确定性,CeIII-MnII 的能量传递机制仍存在争议。在此,我们首次探索了两种具有清晰晶体结构的设计良好的发光异核配合物(即 Ce-N8-MnII 和 CeIII-MnII)的能量传递机制。即 Ce-N8-Mn 和 Ce-N2O6-Mn (N8 = 1,4,7,10,13,16,21,24-octaazabicyclo[8.8.8]hexacosane; N2O6 = 4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane )。金属中心之间的短距离促进了这两种配合物中 CeIII 向 MnII 的有效能量转移,从而使光致发光量子产率高达一倍。经过对这两种异核配合物以及两种参考配合物 Ce(N8)Br3 和 Ce(N2O6)Br3 的系统研究,我们得出结论:偶极子-四极相互作用是异核配合物中最主要的能量转移机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
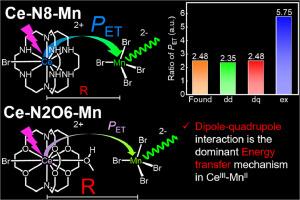
Direct identification of energy transfer mechanism in CeIII-MnII system by constructing molecular heteronuclear complexes
Sensitization of metal-centered forbidden transitions is of great significance. Solid MnII-based phosphors with d-d forbidden transition sensitized by CeIII with d-f allowed transition are promising light conversion materials, but the energy transfer mechanism in CeIII-MnII is still in dispute for the uncertainty of distances between metal centers. Herein, for the first time, we explored the energy transfer mechanism in two well-designed luminescent heteronuclear complexes with clear crystal structures, i.e. Ce-N8-Mn and Ce-N2O6-Mn (N8 = 1,4,7,10,13,16,21,24-octaazabicyclo[8.8.8]hexacosane; N2O6 = 4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane). Short distances between metal centers facilitate efficient energy transfer from CeIII to MnII in both complexes, resulting in high photoluminescence quantum yield up to unity. After systematic study of the two heteronuclear complexes as well as two reference complexes Ce(N8)Br3 and Ce(N2O6)Br3, we concluded that dipole-quadrupole interaction is the dominant energy transfer mechanism in the heteronuclear complexes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


