为区域特异性 O-硫酸化预设的软骨素硫酸二糖亚型的适应性合成
IF 2.5
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
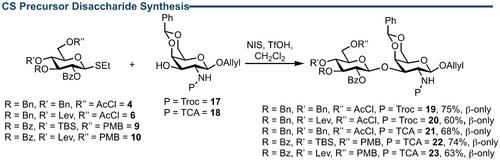
硫酸软骨素(CS)双糖前体(包括 CS-D 等稀有亚型)的不同合成路线已经开发出来。糖基化后氧化成 D-葡萄糖醛酸和正交保护基团可获得 CS-A、CS-C、CS-D、CS-E 和 CS-O 前体亚型。更值得注意的是,使用二氯苯基硼烷(PhBCl2)和三乙基硅烷(Et3SiH)对受 4,6-O-亚苄基保护的二糖进行 4-O-苄基区域选择性还原开环,两步即可获得 CS-D 前体,收率为 73%。最后,还完成了含有可共轭异构烯丙基醚的 6-O 磺化 CS-C 二糖的合成。这些材料将为进一步合成和研究软骨素硫酸盐提供一个基准。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Adaptable Synthesis of Chondroitin Sulfate Disaccharide Subtypes Preprogrammed for Regiospecific O‐Sulfation
A divergent synthetic route to chondroitin sulfate (CS) disaccharide precursors, including rarer subtypes such as CS−D, has been developed. From common intermediates, a series of thioglycoside D‐glucosyl donors and 4,6‐O‐benzylidene protected D‐galactosamine acceptors are utilised in a robust glycosylation reaction, achieving β‐selectivity and consistent yields (60–75 %) on scales >2.0 g. A post‐glycosylation oxidation to D‐glucuronic acid and orthogonal protecting groups delivers access to CS−A, CS−C, CS−D, CS−E and CS−O precursor subtypes. Of further note is a 4‐O‐benzyl regioselective reductive ring opening of a 4,6‐O‐benzylidene protected disaccharide using dichlorophenylborane (PhBCl2) and triethylsilane (Et3SiH) to access a CS−D precursor, in 73 % yield over two steps. Finally, synthesis of a 6‐O‐sulfated CS−C disaccharide containing a conjugable anomeric allyl tether is completed. These materials will provide a benchmark to further synthesise and study chondroitin sulfates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


