通过社区根除幽门螺旋杆菌预防胃癌:分组随机对照试验
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
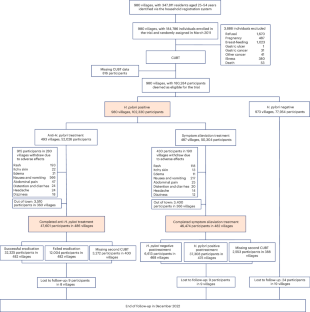
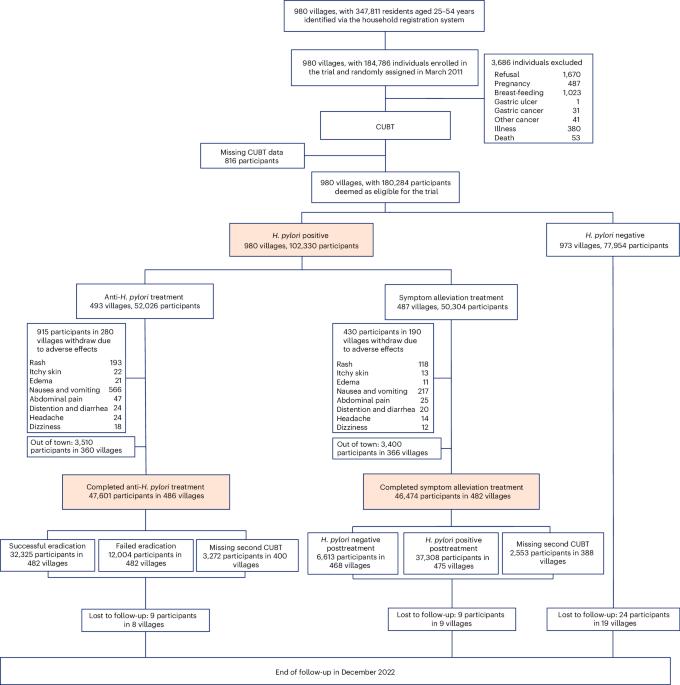
胃癌是中国癌症相关死亡的主要原因。幽门螺杆菌影响着全球 40% 以上的人口,是胃癌的主要风险因素。尽管之前的临床试验表明,根除幽门螺杆菌可以降低胃癌风险,但这一点仍有待采用基于人群的方法来证明。我们在中国临朐县开展了一项以社区为基础的群组随机对照优效干预试验,通过 13C- 尿素呼气试验检测出幽门螺杆菌呈阳性的患者被随机分配到以下两种治疗方案中:(1) 接受为期 10 天的四联抗幽门螺杆菌治疗(包括 20 毫克的 20C- 尿素呼气试验);(2) 接受为期 10 天的四联抗幽门螺杆菌治疗。幽门螺杆菌治疗(包括 20 毫克奥美拉唑、750 毫克四环素、400 毫克甲硝唑和 300 毫克枸橼酸铋)或 (2) 每天服用一次奥美拉唑和枸橼酸铋以缓解症状。幽门螺杆菌阴性者不接受任何治疗。我们将胃癌发病率作为主要研究结果。在长达 11.8 年的随访过程中,共有来自 980 个村庄的 180,284 名符合条件的参与者参与其中,共记录了 1,035 例胃癌病例。在意向治疗分析中,接受抗幽门螺杆菌治疗的患者胃癌发病率略有下降(危险比为0.86,95%置信区间为0.74-0.99),与治疗失败者相比,成功根除幽门螺杆菌者的效果更明显(危险比为0.81,95%置信区间为0.69-0.96)。在为期 10 天的治疗中,1345 名参与者出现了中度不良反应。在治疗或随访期间,我们均未观察到严重的不可耐受不良反应。研究结果表明,幽门螺杆菌的大规模筛查和根除有可能成为预防胃癌的一项公共卫生政策。中国临床试验注册中心标识符:ChiCTR-TRC-10000979。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Gastric cancer prevention by community eradication of Helicobacter pylori: a cluster-randomized controlled trial
Gastric cancer is a leading cause of cancer-related deaths in China. Affecting more than 40% of the world’s population, Helicobacter pylori is a major risk factor for gastric cancer. While previous clinical trials indicated that eradication of H. pylori could reduce gastric cancer risk, this remains to be shown using a population-based approach. We conducted a community-based, cluster-randomized, controlled, superiority intervention trial in Linqu County, China, with individuals who tested positive for H. pylori using a 13C-urea breath test randomly assigned to receiving either (1) a 10-day, quadruple anti-H. pylori treatment (comprising 20 mg of omeprazole, 750 mg of tetracycline, 400 mg of metronidazole and 300 mg of bismuth citrate) or (2) symptom alleviation treatment with a single daily dosage of omeprazole and bismuth citrate. H. pylori-negative individuals did not receive any treatment. We examined the incidence of gastric cancer as the primary outcome. A total of 180,284 eligible participants from 980 villages were enrolled over 11.8 years of follow-up, and a total of 1,035 cases of incident gastric cancer were documented. Individuals receiving anti-H. pylori therapy showed a modest reduction in gastric cancer incidence in intention-to-treat analyses (hazard ratio 0.86, 95% confidence interval 0.74–0.99), with a stronger effect observed for those having successful H. pylori eradication (hazard ratio 0.81, 95% confidence interval 0.69–0.96) than for those who failed treatment. Moderate adverse effects were reported in 1,345 participants during the 10-day treatment. We observed no severe intolerable adverse events during either treatment or follow-up. The findings suggest the potential for H. pylori mass screening and eradication as a public health policy for gastric cancer prevention. Chinese Clinical Trial Registry identifier: ChiCTR-TRC-10000979 . A cluster-randomized trial carried out across 980 villages in a high-risk region in China found that systematic treatment of antibiotics, omeprazole and bismuth modestly reduced gastric cancer incidence in Helicobacter pylori-positive populations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


