红光供电的硅纳米线生物光化学二极管,可同时实现二氧化碳还原和甘油增值
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
无偏压光化学二极管(p 型光电阴极与 n 型光电阳极相连,利用光驱动光电化学还原和氧化对)是实现光驱动二氧化碳燃料发电的平台。然而,阴极二氧化碳还原与阳极氧进化反应(OER)相结合的传统设计需要大量的能量输入。在这里,我们提出了一种光化学二极管装置,利用红光(740 纳米)同时驱动生物光阴极二氧化碳到多碳的转化和光阳极甘油氧化反应,以替代 OER,从而克服上述热力学限制。该装置由高效二氧化碳固定微生物 Sporomusa ovata 与硅纳米线光电阴极和铂-金负载硅纳米线光阳极连接组成。这种光化学二极管可在低强度(20 mW cm-2)红光照射下无偏压运行,阴极和阳极产物的法拉第效率约为 80%。这项工作提供了另一种光合作用途径,可减少过量的二氧化碳排放,并从二氧化碳和甘油中高效生成增值化学品。本文章由计算机程序翻译,如有差异,请以英文原文为准。
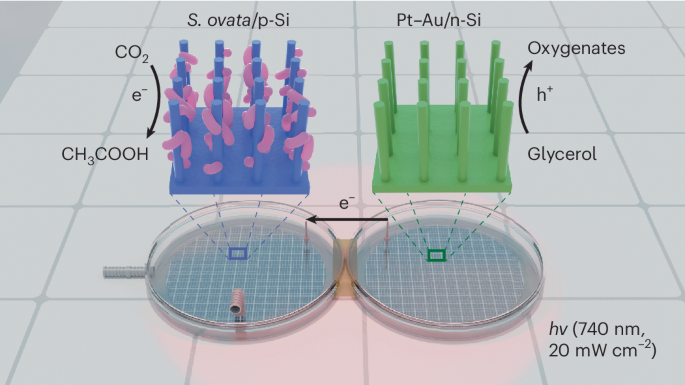
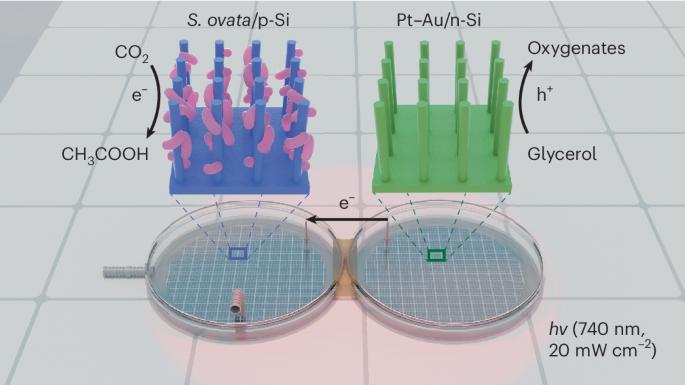
A red-light-powered silicon nanowire biophotochemical diode for simultaneous CO2 reduction and glycerol valorization
A bias-free photochemical diode, in which a p-type photocathode is connected to an n-type photoanode to harness light for driving photoelectrochemical reduction and oxidation pairs, serves as a platform for realizing light-driven fuel generation from CO2. However, the conventional design, in which cathodic CO2 reduction is coupled with the anodic oxygen evolution reaction (OER), requires substantial energy input. Here we present a photochemical diode device that harnesses red light (740 nm) to simultaneously drive biophotocathodic CO2-to-multicarbon conversion and photoanodic glycerol oxidation as an alternative to the OER to overcome the above thermodynamic limitation. The device consists of an efficient CO2-fixing microorganism, Sporomusa ovata, interfaced with a silicon nanowire photocathode and a Pt–Au-loaded silicon nanowire photoanode. This photochemical diode operates bias-free under low-intensity (20 mW cm−2) red light irradiation with ~80% Faradaic efficiency for both the cathodic and anodic products. This work provides an alternative photosynthetic route to mitigate excessive CO2 emissions and efficiently generate value-added chemicals from CO2 and glycerol. CO2 reduction to value-added products is an attractive strategy in sustainable chemistry. Now a photochemical diode simultaneously drives biophotocathodic CO2-to-multicarbon conversion and photoanodic glycerol oxidation under red light with a photocurrent density of ~1.2 mA cm−2 and a Faradaic efficiency of ~80%.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


