用于设计无细胞蛋白质展开和降解途径的模块化 DNA 折纸纳米隔室
IF 38.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
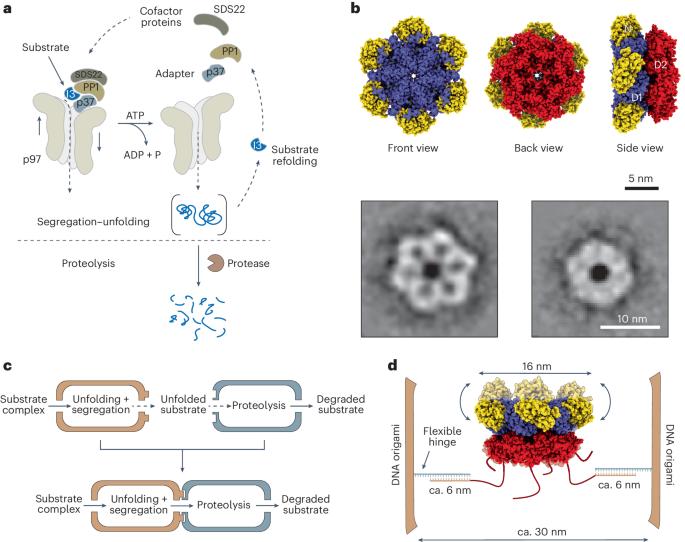
在细胞内,化学反应通常通过模块化结构进行限制和组织。这有利于分子物种的定向定位及其高效转运到后续部位。在这里,我们介绍了一种无细胞纳米级模型,它利用区隔策略来进行受控蛋白质的展开和降解。我们的合成模型由两个相连的 DNA 折纸纳米小室组成(每个小室的尺寸为 25 nm × 41 nm × 53 nm):其中一个小室中装有蛋白质展开机 p97,另一个小室中装有蛋白酶糜蛋白酶。我们实现了 p97 在第一区室中的单向固定,建立了一个控制第二区室中底物招募、转运和加工的网关机制。我们的数据显示,虽然空间限制使单个反应的速率提高了 10 倍,但将隔室化的酶物理连接成嵌合体可有效地耦合两个反应,并将脱靶蛋白水解率降低近 6 倍。因此,我们的模块化方法可作为人工纳米工厂的工程蓝图,其催化性能和功能的重塑将超越自然系统。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A modular DNA origami nanocompartment for engineering a cell-free, protein unfolding and degradation pathway
Within the cell, chemical reactions are often confined and organized through a modular architecture. This facilitates the targeted localization of molecular species and their efficient translocation to subsequent sites. Here we present a cell-free nanoscale model that exploits compartmentalization strategies to carry out regulated protein unfolding and degradation. Our synthetic model comprises two connected DNA origami nanocompartments (each measuring 25 nm × 41 nm × 53 nm): one containing the protein unfolding machine, p97, and the other housing the protease chymotrypsin. We achieve the unidirectional immobilization of p97 within the first compartment, establishing a gateway mechanism that controls substrate recruitment, translocation and processing within the second compartment. Our data show that, whereas spatial confinement increases the rate of the individual reactions by up to tenfold, the physical connection of the compartmentalized enzymes into a chimera efficiently couples the two reactions and reduces off-target proteolysis by almost sixfold. Hence, our modular approach may serve as a blueprint for engineering artificial nanofactories with reshaped catalytic performance and functionalities beyond those observed in natural systems. This study presents DNA-origami biocatalytic modular nanocompartments for programmed regulation of protein unfolding and degradation. These artificial nanofactories augment reaction kinetics, improve enzyme performance and reduce off-target effects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


