脂肽巴基洛替林 C 的全合成发现促进自噬的强效抗癌同系物
IF 4
3区 医学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
我们开发出了一种聚合策略,首次全合成了脂肽双醋菊酯 C。该合成的主要特征包括 Crimmins 醋酸醛醇、Steglich 酯化和大环内酰胺化。经过系统的结构-活性关系研究,制备出该天然产物的 29 种变体,其中一些设计的类似物对多种人类癌细胞株显示出良好的细胞毒性作用。其中最有效的类似物在亚摩尔剂量下对三阴性乳腺癌(MDA-MB-231)细胞系的细胞毒性比百乐甜素 C 增强了 37 倍。研究还发现,一些类似物能诱导癌细胞发生自噬,甚至导致癌细胞死亡,其剂量远远低于已知的自噬诱导肽。研究结果表明,适当改良百绿苷 C 的化学合成在新型抗癌化疗药物的开发中发挥着重要作用,这将使未来在此模板上合理设计新型自噬诱导剂成为可能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
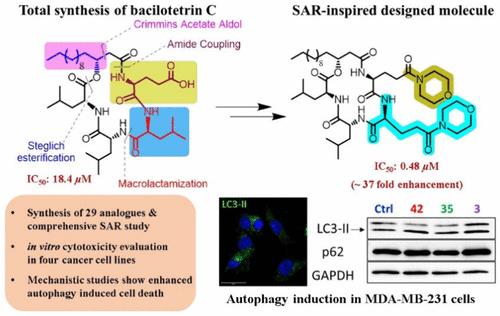
Total Synthesis of Lipopeptide Bacilotetrin C: Discovery of Potent Anticancer Congeners Promoting Autophagy
A convergent strategy for the first total synthesis of the lipopeptide bacilotetrin C has been developed. The key features of this synthesis include Crimmins acetate aldol, Steglich esterification, and macrolactamization. Twenty-nine variants of the natural product were prepared following a systematic structure–activity relationship study, where some of the designed analogues showed promising cytotoxic effects against multiple human carcinoma cell lines. The most potent analogue exhibited a ∼37-fold enhancement in cytotoxicity compared to bacilotetrin C in a triple-negative breast cancer (MDA-MB-231) cell line at submicromolar doses. The study further revealed that some of the analogues induced autophagy in cancer cells to the point of their demise at doses much lower than those of known autophagy-inducing peptides. The results demonstrated that the chemical synthesis of bacilotetrin C with suitable improvisation plays an important role in the development of novel anticancer chemotherapeutics, which would allow future rational design of novel autophagy inducers on this template.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
ACS Medicinal Chemistry Letters
CHEMISTRY, MEDICINAL-
CiteScore
7.30
自引率
2.40%
发文量
328
审稿时长
1 months
期刊介绍:
ACS Medicinal Chemistry Letters is interested in receiving manuscripts that discuss various aspects of medicinal chemistry. The journal will publish studies that pertain to a broad range of subject matter, including compound design and optimization, biological evaluation, drug delivery, imaging agents, and pharmacology of both small and large bioactive molecules. Specific areas include but are not limited to:
Identification, synthesis, and optimization of lead biologically active molecules and drugs (small molecules and biologics)
Biological characterization of new molecular entities in the context of drug discovery
Computational, cheminformatics, and structural studies for the identification or SAR analysis of bioactive molecules, ligands and their targets, etc.
Novel and improved methodologies, including radiation biochemistry, with broad application to medicinal chemistry
Discovery technologies for biologically active molecules from both synthetic and natural (plant and other) sources
Pharmacokinetic/pharmacodynamic studies that address mechanisms underlying drug disposition and response
Pharmacogenetic and pharmacogenomic studies used to enhance drug design and the translation of medicinal chemistry into the clinic
Mechanistic drug metabolism and regulation of metabolic enzyme gene expression
Chemistry patents relevant to the medicinal chemistry field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


