烯基硼酸酯的辐射聚合和 C-B 键转化:通过侧链置换合成聚合物,克服合成限制
IF 2.3
4区 化学
Q3 POLYMER SCIENCE
引用次数: 0
摘要
乙烯基聚合物通常是通过相应乙烯基化合物的加成聚合反应合成的。然而,聚合能力在很大程度上取决于乙烯基上的取代基,从而导致乙烯基聚合物的分子结构存在各种合成限制。鉴于社会对增强聚合物材料性能和功能的需求日益增长,需要创新的合成技术通过灵活的分子设计来开发新一代聚合物。作者通过使用烯基硼酸盐作为自由基聚合的单体,为克服聚合物合成中的这些限制做出了巨大努力。由此得到的聚合物主链上含有硼,在聚合后转化过程中,通过碳-硼键的裂解,可以用其他元素取代硼侧链。这种以 "侧链置换 "为基础的策略使得以前无法合成的各种聚合物得以合成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
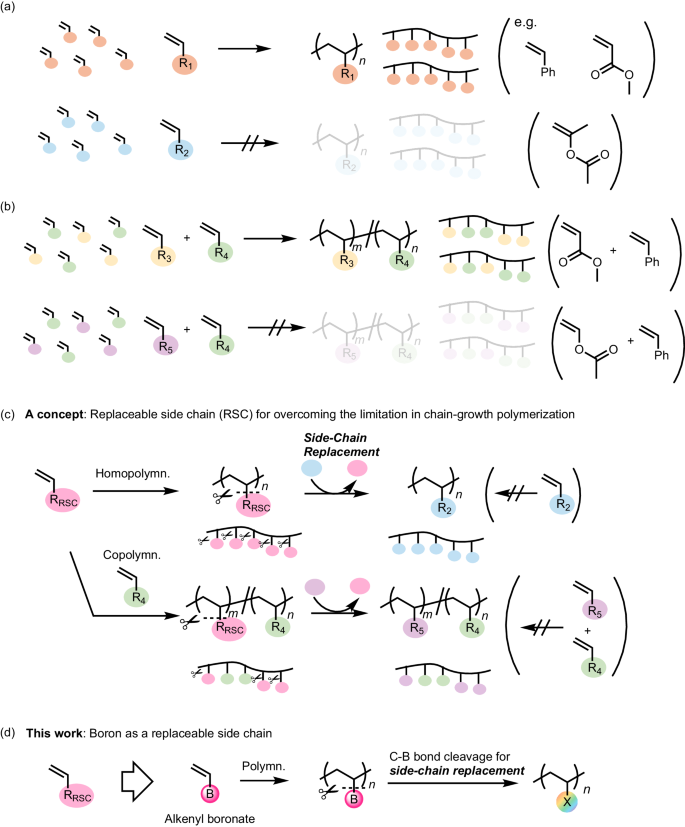
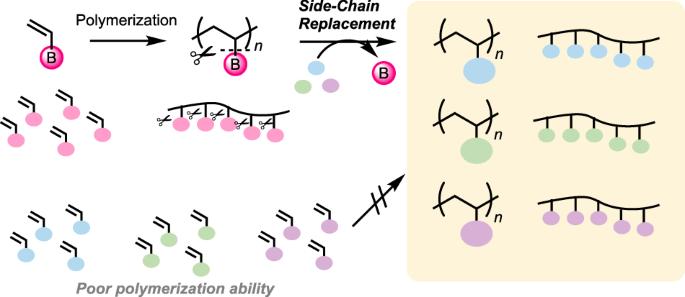
Radical polymerization of alkenyl boronates and C–B bond transformation: polymer synthesis through side-chain replacement for overcoming synthetic limitations
Vinyl polymers are typically synthesized through the addition polymerization of corresponding vinyl compounds. However, the polymerization ability significantly depends on the substituent on the vinyl moiety, resulting in various synthetic limitations in the molecular structure of vinyl polymers. Given the increasing societal demand for enhanced properties and functions of polymer materials, innovative synthetic technologies are required for developing next-generation polymers through flexible molecular design. The author has made considerable efforts to overcome these limitations in polymer synthesis by employing alkenyl boronates as monomers for radical polymerization. The resulting polymers bear boron on the main chain, allowing the replacement of boron side chains with other elements through the cleavage of carbon–boron bonds in postpolymerization transformations. This strategy, based on “side-chain replacement,” has enabled the synthesis of various polymers that were previously inaccessible. The review highlights the author’s recent discovery of the radical (co)polymerization ability of alkenylboronic acid derivatives and C–B bond-cleaving side-chain replacement in polymer reaction. The polymerization ability is attributed to the vacant p-orbital of boron, which can stabilize chain-growth radical species. In copolymerization and controlled polymerization, the boron monomer behaves as a relatively electron-rich and conjugated monomer. The boron attached to the polymer main chain was replaceable with other elements, providing access to various polymers of which synthesis is not straightforward, such as poly(α-methyl vinyl alcohol), poly(vinyl alcohol-co-styrene), and poly(ethylene-co-acrylate).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Journal
化学-高分子科学
CiteScore
5.60
自引率
7.10%
发文量
131
审稿时长
2.5 months
期刊介绍:
Polymer Journal promotes research from all aspects of polymer science from anywhere in the world and aims to provide an integrated platform for scientific communication that assists the advancement of polymer science and related fields. The journal publishes Original Articles, Notes, Short Communications and Reviews.
Subject areas and topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Polymer synthesis and reactions
Polymer structures
Physical properties of polymers
Polymer surface and interfaces
Functional polymers
Supramolecular polymers
Self-assembled materials
Biopolymers and bio-related polymer materials
Polymer engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


