镍上硫化钴纳米棒的亚硝酸盐到氨的电还原作用
IF 5.5
2区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
电化学亚硝酸盐还原反应(NO2RR)可满足去除亚硝酸盐(NO2-)污染物的需要,并为生产氨气(NH3)提供了一种可持续的方法。然而,从 NO2- 到 NH3 的转化过程是一个复杂的多步过程,涉及六电子转移,这给设计 NO2RR 的高效催化剂带来了巨大挑战。本文通过相控合成法合成了一系列锚定在不同硫含量泡沫镍(NF)上的硫化钴(CoxSy)纳米棒(NRs)(CoS1.035 NRs/NF、CoS2 NRs/NF、Co3S4 NRs/NF 和 CoS1.097 NRs/NF)。最佳的钴基硫化物纳米棒(CoS2 NRs/NF)在 NO2RR 中表现出卓越的性能,具有极高的 NO2- 转化率(97.8%)、法拉第效率(97.9%)和 NH3 选择性(98.7%)。这些结果超过了之前报道的 NO2RR 催化剂。控制实验证实,对于不同的硫化钴相,硫含量越高,产生的活性氢越多,这更有利于将 NO2- 电还原为 NH3。这项研究为合理设计 NO2RR 催化剂提出了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
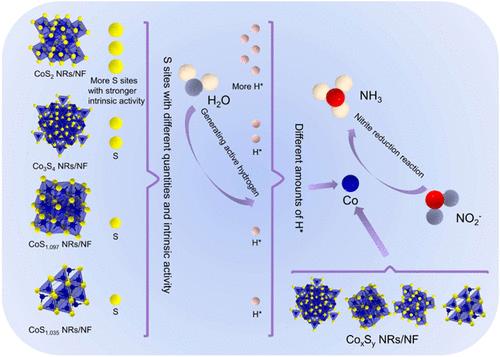
Electroreduction from Nitrite to Ammonia over Cobalt Sulfide Nanorods on Nickel
Electrochemical nitrite reduction reaction (NO2RR) can meet the need to remove nitrite (NO2–) pollutants and provide a sustainable way to produce ammonia (NH3). However, the conversion process from NO2– to NH3 is a complex multistep process involving six-electron transfer, posing a significant challenge for designing efficient catalysts for the NO2RR. Here, a series of cobalt-based sulfide (CoxSy) nanorods (NRs) anchored on nickel foam (NF) with varying sulfur contents (CoS1.035 NRs/NF, CoS2 NRs/NF, Co3S4 NRs/NF, and CoS1.097 NRs/NF) were synthesized through phase-controlled synthesis. The optimal cobalt-based sulfide nanorods (CoS2 NRs/NF) exhibit superior performance in the NO2RR, with an extremely high NO2– conversion rate (97.8%), Faradaic efficiency (97.9%), and NH3 selectivity (98.7%). These results surpass those of previously reported NO2RR catalysts. Controlled experiments confirm that for the different cobalt sulfide phases, the higher the sulfur content, the more active hydrogen produced, which is more conducive to the electroreduction from NO2– to NH3. This research presents novel insight into the rational design of NO2RR catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Nano Materials
Multiple-
CiteScore
8.30
自引率
3.40%
发文量
1601
期刊介绍:
ACS Applied Nano Materials is an interdisciplinary journal publishing original research covering all aspects of engineering, chemistry, physics and biology relevant to applications of nanomaterials. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important applications of nanomaterials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


