二氧化碳和甲醇 Ca(OH)2 分散液反应生成 CaCO3:瞬时甲氧基盐、碳酸酯和溶胶
IF 3.7
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
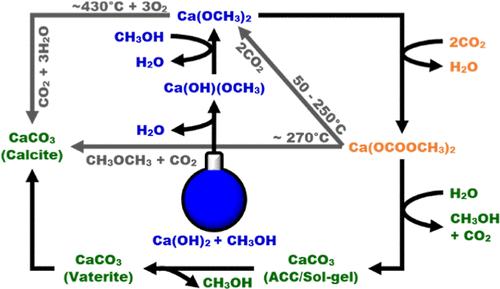
该研究结合原位和原位表征技术,确定了甲醇/水(CH3OH/H2O)体系中氢氧化钙(Ca(OH)2)分散体形成碳酸钙(CaCO3)的机理。中红外(mid-IR)分析表明,在没有二氧化碳(CO2)的情况下,Ca(OH)2 与 CH3OH 建立反应平衡,形成氢氧化钙甲醇(Ca(OH)(OCH3))和甲醇钙(Ca(OCH3)2)。结合原位中红外光谱、热重分析(TGA)、X 射线衍射(XRD)、X 射线吸收光谱和扫描电子显微镜,对二氧化碳存在下形成的反应产物进行检查,发现形成了二甲基碳酸钙(Ca(OCOOCH3)2)。这强烈表明,碳化是通过与 Ca(OH)2 和 CH3OH 反应生成的 Ca(OCH3)2 反应进行的。时间分辨 XRD 显示,在有 H2O 存在的情况下,Ca(OCOOCH3)2 酯释放出 CH3OH 和 CO2,形成 ACC,随后转变成辉石,再转变成方解石。热重分析(TGA)显示,在没有 H2O 的情况下,Ca(OCOOCH3)2 的热分解主要导致 Ca(OCH3)2 的重整,但伴随着一个重要的平行反应,释放出二甲醚(CH3OCH3)和二氧化碳。CaCO3 是这两种分解途径的最终产物。对于 H2O 含量超过 50 摩尔%的 CH3OH/H2O 混合物,尽管在 20 摩尔% 和 40 摩尔% 的 CH3OH 系统的原位中红外光谱中仍能明显看到一些 Ca(OCOOCH3)2 的形成,但由 Ca(OH)2 直接形成方解石已成为主要途径。在≤20 摩尔% H2O 的存在下,酯的水解导致形成 ACC 溶胶凝胶。在 90 和 100 mol % CH3OH 体系中,都观察到了扩散受限的 ACC → vaterite → 方解石转化。还检测到了文石的痕迹。我们认为,这是首次系统地通过实验描述甲醇相中 Ca(OH)2 碳化过程中的这些反应途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Reactive CaCO3 Formation from CO2 and Methanolic Ca(OH)2 Dispersions: Transient Methoxide Salts, Carbonate Esters and Sol–Gels
A combination of ex situ and in situ characterization techniques was used to determine the mechanism of calcium carbonate (CaCO3) formation from calcium hydroxide (Ca(OH)2) dispersions in methanol/water (CH3OH/H2O) systems. Mid-infrared (mid-IR) analysis shows that in the absence of carbon dioxide (CO2) Ca(OH)2 establishes a reaction equilibrium with CH3OH, forming calcium hydroxide methoxide (Ca(OH)(OCH3)) and calcium methoxide (Ca(OCH3)2). Combined ex situ mid-IR, thermogravimetric analysis (TGA), X-ray diffraction (XRD), X-ray absorption spectroscopy and scanning electron microscopy examination of the reaction product formed in the presence of CO2 reveals the formation of calcium dimethylcarbonate (Ca(OCOOCH3)2). This strongly suggests that carbonation takes place by reaction with the Ca(OCH3)2 formed from a Ca(OH)2 and CH3OH reaction. Time-resolved XRD indicates that in the presence of H2O the Ca(OCOOCH3)2 ester releases CH3OH and CO2, forming ACC, which subsequently transforms into vaterite and then calcite. TGA reveals that thermal decomposition of Ca(OCOOCH3)2 in the absence of H2O mainly leads to the reformation of Ca(OCH3)2, but this is accompanied by a significant parallel reaction that releases dimethylether (CH3OCH3) and CO2. CaCO3 is the final product in both decomposition pathways. For CH3OH/H2O mixtures containing more than 50 mol % H2O, direct formation of calcite from Ca(OH)2 becomes the dominant pathway, although the formation of some Ca(OCOOCH3)2 was still evident in the in situ mid-IR spectra of 20 and 40 mol % CH3OH systems. In the presence of ≤20 mol % H2O, hydrolysis of the ester led to the formation of an ACC sol–gel. In both the 90 and 100 mol % CH3OH systems, diffusion-limited ACC → vaterite → calcite transformations were observed. Traces of aragonite were also detected. We believe that this is the first time that these reaction pathways during the carbonation of Ca(OH)2 in a methanolic phase have been systematically and experimentally characterized.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.70
自引率
0.00%
发文量
0
期刊介绍:
ACS Physical Chemistry Au is an open access journal which publishes original fundamental and applied research on all aspects of physical chemistry. The journal publishes new and original experimental computational and theoretical research of interest to physical chemists biophysical chemists chemical physicists physicists material scientists and engineers. An essential criterion for acceptance is that the manuscript provides new physical insight or develops new tools and methods of general interest. Some major topical areas include:Molecules Clusters and Aerosols; Biophysics Biomaterials Liquids and Soft Matter; Energy Materials and Catalysis

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


