可重复使用催化剂 (Bu3Sn)2MoO4 促进 3-芳基甲基吲哚的高效一步合成
IF 1.7
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
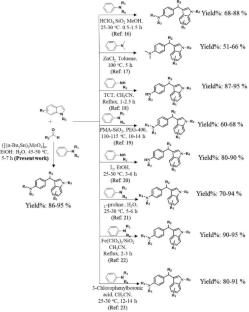
一种高效的多组分合成取代的 3-芳基甲基吲哚衍生物的方案,该方案采用三组分策略,在 45-50°C 温度下用水:乙醇 (H2O:EtOH) 合成,收率良好甚至极佳。该方案的主要优点是反应条件温和,易于操作,催化剂可循环使用多达四个催化周期。图解摘要 简介:以(Bu3Sn)2MoO4 为可重复使用的催化剂,在乙醇:水为溶剂体系中采用高效的多组分策略合成取代的 3-芳基甲基吲哚,产率达到良好至优异水平。该催化剂可重复使用四个催化循环。本文章由计算机程序翻译,如有差异,请以英文原文为准。
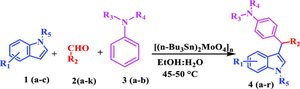
Efficient one-pot synthesis of 3-arylmethyl indoles promoted by (Bu3Sn)2MoO4 as a reusable catalyst
An efficient multi-component protocol for synthesizing substituted 3-arylmethyl indoles derivatives through a three-component strategy using water: ethanol (H2O:EtOH) at 45–50°C with good to excellent yields. The main advantages of this protocol are mild reaction conditions, easy workup, and recyclability of the catalyst for up to four catalytic cycles.
Graphical abstract
Synopsis: Synthesis of substituted 3-arylmethyl indoles by using efficient multi-component strategy in ethanol: water as a solvent system by using (Bu3Sn)2MoO4 as a reusable catalyst at 45–50 °C with good to excellent yields. The catalyst is reusable in four catalytic cycles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Chemical Sciences
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
3.10
自引率
5.90%
发文量
107
审稿时长
1 months
期刊介绍:
Journal of Chemical Sciences is a monthly journal published by the Indian Academy of Sciences. It formed part of the original Proceedings of the Indian Academy of Sciences – Part A, started by the Nobel Laureate Prof C V Raman in 1934, that was split in 1978 into three separate journals. It was renamed as Journal of Chemical Sciences in 2004. The journal publishes original research articles and rapid communications, covering all areas of chemical sciences. A significant feature of the journal is its special issues, brought out from time to time, devoted to conference symposia/proceedings in frontier areas of the subject, held not only in India but also in other countries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


