以 SiO2 为载体的 DMDAAC 改性杂多酸作为高活性、高稳定性的甲基丙烯醛氧化催化剂
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
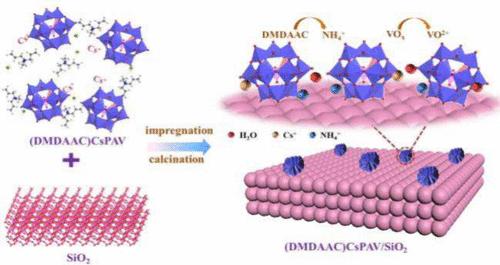
鉴于 CsPAV 比表面积小、热稳定性差、易分解等缺点,有机铵源二甲基二烯丙基氯化铵(DMDAAC)对其进行了改性。通过浸渍法制备了一系列具有不同 (DMDAAC)CsPAV 负载的 (DMDAAC)CsPAV/SiO2 催化剂。当 DMDAAC 改性 CsPAV 被支撑在 SiO2 上时,它表现出较高的比表面积和更多的活性位点。通过将甲基丙烯醛(MAL)氧化为甲基丙烯酸(MAA),研究了 (DMDAAC)CsPAV/SiO2 的催化性能。傅立叶变换红外光谱(FT-IR)、X射线衍射(XRD)、热重分析(TG)、NH3-TPD、核磁共振(NMR)和 XPS 对催化剂的性能进行了表征。SiO2 与 (DMDAAC)CsPAV 之间存在相互作用,DMDAAC 在煅烧过程中形成 NH4+ 结晶盐。形成的 NH4+ 与 SiO2 载体之间的相互作用阻止了 CsPAV 的分解。(DMDAAC)CsPAV/SiO2表现出较高的稳定性,在长期评估试验中催化性能稳定。在最佳条件下,50(DMDAAC)CsPAV/SiO2 的 MAL 转化率为 80.8%,对 MAA 的选择性为 89.1%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
SiO2-Supported DMDAAC-Modified Heteropoly Acid as Highly Active and Stability Catalysts for Methacrolein Oxidation
In view of the drawbacks of small specific surface area, poor thermal stability, and easy decomposition, CsPAV was modified by the organic ammonium source dimethyl diallyl ammonium chloride (DMDAAC). A series of (DMDAAC)CsPAV/SiO2 catalysts with different (DMDAAC)CsPAV loadings were prepared by an impregnation method. When DMDAAC-modified CsPAV was supported on SiO2, it exhibited a high specific surface area and more active sites. The catalytic performance of (DMDAAC)CsPAV/SiO2 was investigated by methacrolein (MAL) oxidation to methacrylic acid (MAA). The performance of the catalyst was characterized by FT-IR, XRD, TG, NH3-TPD, NMR, and XPS. There was an interaction between SiO2 and (DMDAAC)CsPAV, and the NH4+ crystalline salt was formed in the process of calcination from DMDAAC. The interaction between the formed NH4+ and SiO2 carrier prevented the decomposition of CsPAV. (DMDAAC)CsPAV/SiO2 showed high stability, and the catalytic performance was stable in the long-term evaluation test. Under the optimum conditions, the conversion of MAL was 80.8%, and the selectivity to MAA was 89.1% for 50(DMDAAC)CsPAV/SiO2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


