脊椎动物基部进化出的胚泡干细胞和神经嵴干细胞的共同特征
IF 13.9
1区 生物学
Q1 ECOLOGY
引用次数: 0
摘要
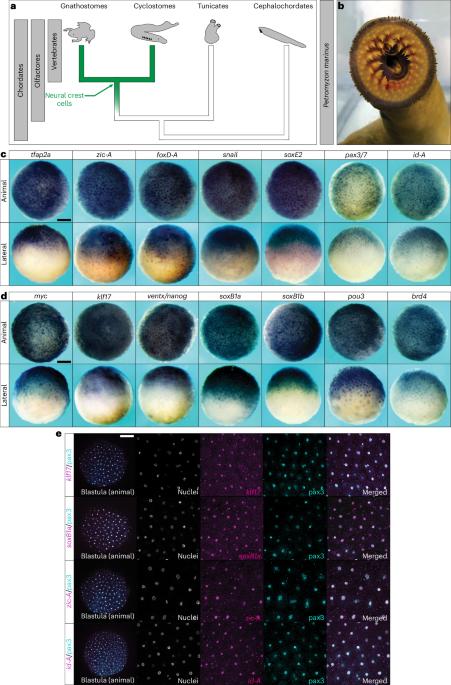
神经嵴是脊椎动物特有的干细胞群,有助于推动脊椎动物的起源和进化。这些细胞的一个显著特点是具有多胚层潜能,这与另一种干细胞群--脊椎动物胚泡的多能干细胞相似。在这里,我们通过比较有颌脊椎动物章鱼和无颌脊椎动物灯鱼的神经嵴和多能性基因调控网络,研究了神经嵴潜能的进化起源。我们揭示了这些基因调控网络中共享调控因子的古老进化起源,可以追溯到现存脊椎动物的最后一个共同祖先。我们重点研究了多能性关键因子pou5,发现灯鱼的pou5直向同源物在动物极细胞中表达,但在神经嵴中却不表达。灯鱼和爪蟾的pou5都能促进神经嵴的形成,这表明pou5的活性是从无颌脊椎动物的神经嵴中丢失的,或者是沿着有颌脊椎动物的茎获得的。最后,我们提供的证据表明,pou5是从类似于祖先 pou3 的支系进化而来,并获得了新的增强神经嵴的活性。这项工作提供的证据表明,神经嵴和胚泡多能性网络都产生于脊椎动物的基部,这可能与pou5的功能进化有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Shared features of blastula and neural crest stem cells evolved at the base of vertebrates
The neural crest is a vertebrate-specific stem cell population that helped drive the origin and evolution of vertebrates. A distinguishing feature of these cells is their multi-germ layer potential, which has parallels to another stem cell population—pluripotent stem cells of the vertebrate blastula. Here, we investigate the evolutionary origins of neural crest potential by comparing neural crest and pluripotency gene regulatory networks of a jawed vertebrate, Xenopus, and a jawless vertebrate, lamprey. We reveal an ancient evolutionary origin of shared regulatory factors in these gene regulatory networks that dates to the last common ancestor of extant vertebrates. Focusing on the key pluripotency factor pou5, we show that a lamprey pou5 orthologue is expressed in animal pole cells but is absent from neural crest. Both lamprey and Xenopus pou5 promote neural crest formation, suggesting that pou5 activity was lost from the neural crest of jawless vertebrates or acquired along the jawed vertebrate stem. Finally, we provide evidence that pou5 acquired novel, neural crest-enhancing activity after evolving from an ancestral pou3-like clade. This work provides evidence that both the neural crest and blastula pluripotency networks arose at the base of the vertebrates and that this may be linked to functional evolution of pou5. Comparison of neural crest and pluripotency gene regulatory networks of Xenopus and lamprey reveals shared regulatory factors in the last common ancestor of extant vertebrates and suggests common molecular features of blastula and neural crest stem cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature ecology & evolution
Agricultural and Biological Sciences-Ecology, Evolution, Behavior and Systematics
CiteScore
22.20
自引率
2.40%
发文量
282
期刊介绍:
Nature Ecology & Evolution is interested in the full spectrum of ecological and evolutionary biology, encompassing approaches at the molecular, organismal, population, community and ecosystem levels, as well as relevant parts of the social sciences. Nature Ecology & Evolution provides a place where all researchers and policymakers interested in all aspects of life's diversity can come together to learn about the most accomplished and significant advances in the field and to discuss topical issues. An online-only monthly journal, our broad scope ensures that the research published reaches the widest possible audience of scientists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


