一种由 TNIP1 驱动、IgG4 升高的系统性自身免疫疾病
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
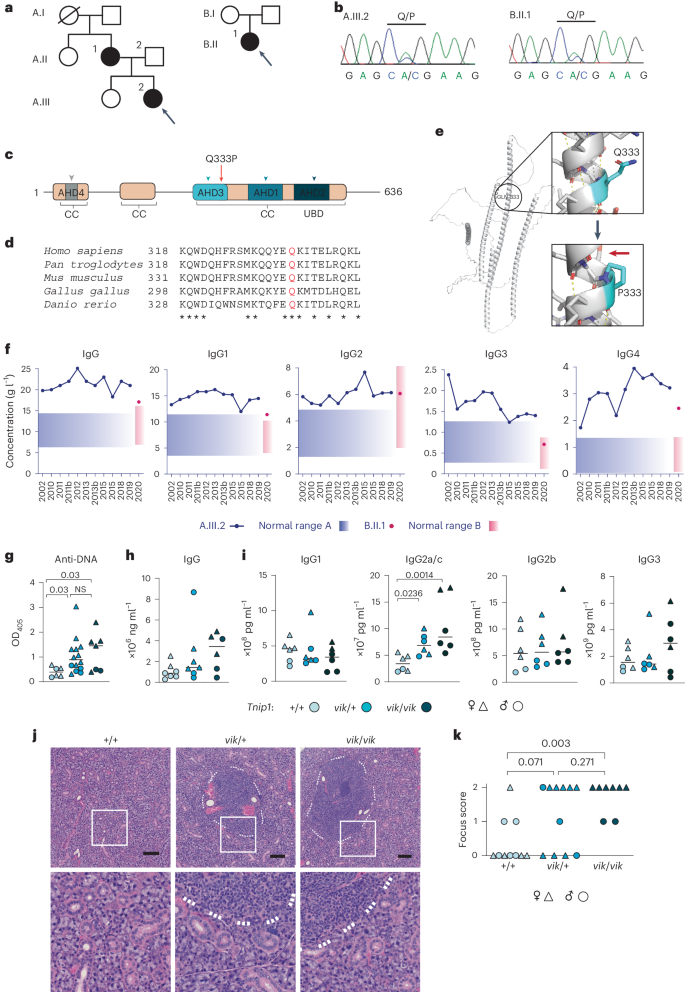
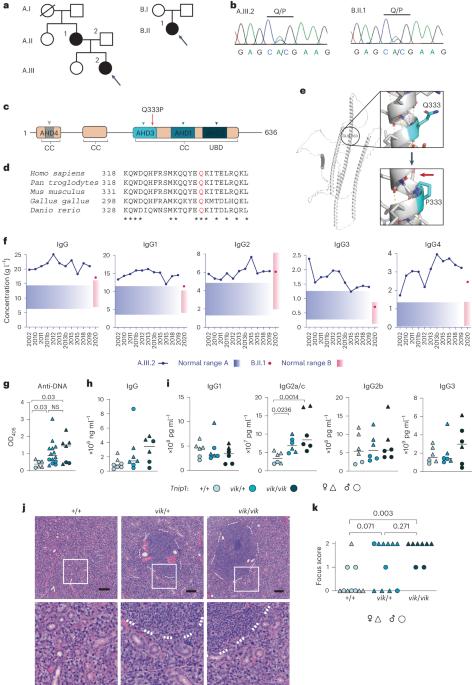
对患有全身性自身免疫性疾病的两个无血缘关系的家族进行的全基因组测序发现,抗核抗体和 IgG4 升高的 TNIP1Q333P 突变体与该疾病具有相同的杂合性。具有同源Q346P变异体的小鼠出现了抗核自身抗体、唾液腺炎症、IgG2c升高、自发性生殖中心以及年龄相关B细胞、浆细胞和滤泡及滤泡外辅助T细胞的扩增。B 细胞表型是细胞自主的,并可通过消减 Toll 样受体 7 (TLR7) 或 MyD88 得到挽救。该变异体增加了干扰素-β,但没有改变活化B细胞的核因子卡巴轻链增强子信号转导,并阻碍了MyD88和IRAK1招募到自噬体。此外,Q333P 变体还影响 TNIP1 在受损线粒体上的定位和有丝分裂吞噬体的形成。在 Tnip1Q346P 小鼠的唾液上皮细胞中,受损的线粒体非常丰富。这些研究结果表明,TNIP1介导的自身免疫可能是由于下游信号分子和受损线粒体招募到自噬体的能力受损导致TLR7信号增强的结果,因此可能会对TLR7靶向疗法产生反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A TNIP1-driven systemic autoimmune disorder with elevated IgG4
Whole-exome sequencing of two unrelated kindreds with systemic autoimmune disease featuring antinuclear antibodies with IgG4 elevation uncovered an identical ultrarare heterozygous TNIP1Q333P variant segregating with disease. Mice with the orthologous Q346P variant developed antinuclear autoantibodies, salivary gland inflammation, elevated IgG2c, spontaneous germinal centers and expansion of age-associated B cells, plasma cells and follicular and extrafollicular helper T cells. B cell phenotypes were cell-autonomous and rescued by ablation of Toll-like receptor 7 (TLR7) or MyD88. The variant increased interferon-β without altering nuclear factor kappa-light-chain-enhancer of activated B cells signaling, and impaired MyD88 and IRAK1 recruitment to autophagosomes. Additionally, the Q333P variant impaired TNIP1 localization to damaged mitochondria and mitophagosome formation. Damaged mitochondria were abundant in the salivary epithelial cells of Tnip1Q346P mice. These findings suggest that TNIP1-mediated autoimmunity may be a consequence of increased TLR7 signaling due to impaired recruitment of downstream signaling molecules and damaged mitochondria to autophagosomes and may thus respond to TLR7-targeted therapeutics. Vinuesa et al. identify patients with systemic autoimmunity and a TNIP1 variant that result in dysregulated B cell function. Mice with the orthologous Tnip1 mutation develop spontaneous autoimmunity associated with impaired mitophagy and autophagic silencing of proteins downstream of Toll-like receptor 7 signaling.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


