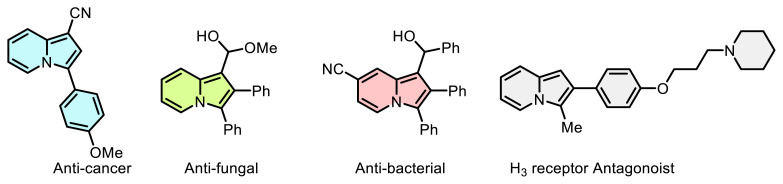
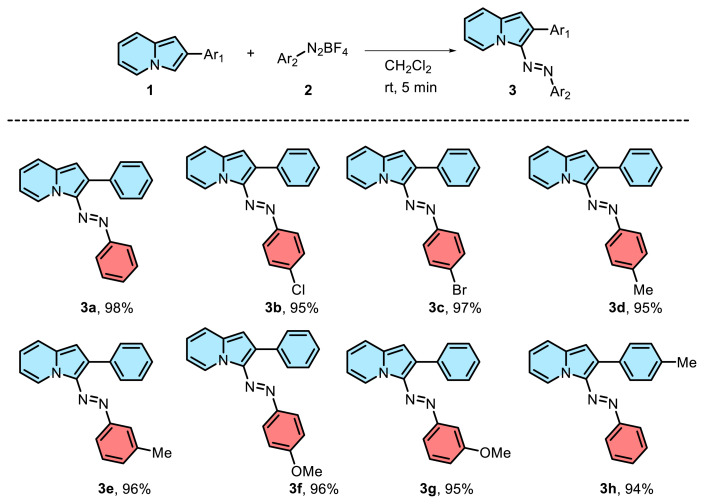
通过芳基偶氮盐快速合成偶氮吲嗪衍生物。
IF 1.4
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
Turkish Journal of Chemistry
Pub Date : 2024-03-11
eCollection Date: 2024-01-01
DOI:10.55730/1300-0527.3675
引用次数: 0
摘要
报告了一种通过芳基偶氮盐合成偶氮吲嗪衍生物的实用、快速和高效的方法,且收率极高。首先,通过简单快速的方法合成了相应的苯胺衍生物。然后,使用多种原生和非原生溶剂研究了最佳反应条件,证明了该方法的稳健性。最后,拓展了该方法对各种来源的吲哚利嗪和苯基二氮四氟硼酸盐的适用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Rapid synthesis of azoindolizine derivatives via aryldiazonium salts.
A practical, rapid, and efficient method for the synthesis of azoindolizine derivatives via aryldiazonium salts with excellent yields was reported. Firstly, the corresponding aniline derivatives were synthesized via a simple and rapid method. Then, the optimal reaction conditions were investigated using a variety of protic and aprotic solvents that demonstrating the robustness of the approach. Finally, the applicability of this method to various sources of indolizine and phenyldiazonium tetrafluoroborate salts was expanded.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Turkish Journal of Chemistry
化学-工程:化工
CiteScore
2.40
自引率
7.10%
发文量
87
审稿时长
3 months
期刊介绍:
The Turkish Journal of Chemistry is a bimonthly multidisciplinary journal published by the Scientific and Technological Research Council of Turkey (TÜBİTAK).
The journal is dedicated to dissemination of knowledge in all disciplines of chemistry (organic, inorganic, physical, polymeric, technical, theoretical and analytical chemistry) as well as research at the interface with other sciences especially in chemical engineering where molecular aspects are key to the findings.
The journal accepts English-language original manuscripts and contribution is open to researchers of all nationalities.
The journal publishes refereed original papers, reviews, letters to editor and issues devoted to special fields.
All manuscripts are peer-reviewed and electronic processing ensures accurate reproduction of text and data, plus publication times as short as possible.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


