利用纳米级电化反应实现选择性氢化催化
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本研究通过模仿纳米级电偶反应,重点优化催化加氢反应,引入两个空间上分离的位点,将 H2 活化为质子和电子对,并选择性还原 -NO2。催化剂系统的设计是在导电碳纳米管上共沉积铂和铁氧体纳米粒子,从而建立电子传递途径。质子溶剂有利于质子传输。用氨或胺修饰的铂上的 H2 分子活化成质子和电子对后,这些活性物质会有效地转移到 Fe2O3 纳米粒子上,从而选择性地将 -NO2 还原成胺,而不会影响其他官能团。与易于氢化 4-硝基苯乙烯的 C=C 和 -NO2 基团的 Pt/CNT 催化剂相比,经 NH3 修饰的 Pt&Fe2O3/CNT 催化剂在 -NO2 氢化方面表现出更高的活性和选择性。在电化学上,铂是氢氧化反应的阳极,而 Fe2O3 则是阴极,可选择性地还原 -NO2。本文章由计算机程序翻译,如有差异,请以英文原文为准。
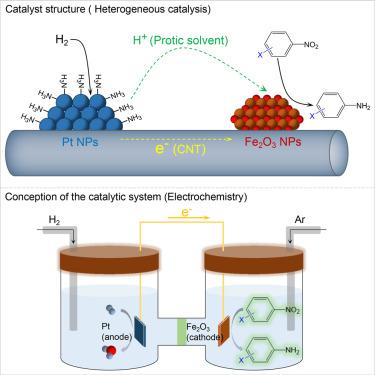
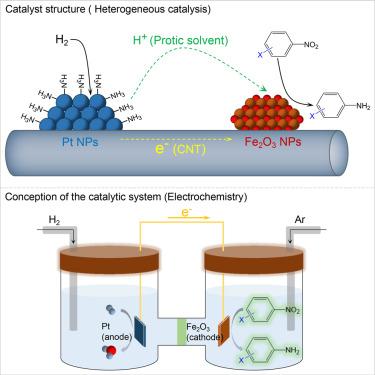
Selective hydrogenation catalysis enabled by nanoscale galvanic reactions
By mimicking nanoscale galvanic reactions, this study focuses on optimizing catalytic hydrogenation by introducing two spatially separated sites for the activation of H2 into proton and electron pairs and the selective reduction of –NO2. The catalyst system is designed with the co-deposition of Pt and Fe2O3 nanoparticles on conductive carbon nanotubes, establishing an electron-transferring pathway. Protic solvents facilitate proton transport. Upon activation of H2 molecules into proton and electron pairs on Pt, modified with ammonia or amines, these active species are efficiently transferred to Fe2O3 nanoparticles for the selective reduction of –NO2 into amines without affecting other functional groups. Compared with Pt/CNT, which easily hydrogenates both C=C and –NO2 groups of 4-nitrostyrene, the Pt&Fe2O3/CNT catalyst modified by NH3 exhibits higher activity and selectivity for –NO2 hydrogenation. Electrochemically, Pt functions as the anode for the hydrogen oxidation reaction, while Fe2O3 acts as the cathode, selectively reducing –NO2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


