部分融合的 12-卟啉纳米中的电荷析出和全局芳香性
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
芳香族和反芳香族环电流可以揭示π共轭大环圆周上的全局电子析出,但这些现象在大环中却鲜为人知。在这里,我们介绍了一种模板指导合成的全π共轭环卟啉 12-mer,它由六个β、介、β边融合的卟啉二聚体组成,通过六个丁二炔桥连接。这种部分融合的纳米卟啉的最低能量π-π∗吸收带远远偏移到了近红外波段,证实了在大环的圆周上存在很强的π共轭作用。通过 1H 和 19F NMR 光谱对氧化和还原纳米环-模板复合物的研究表明,存在相干的全局(反)芳香环电流,这与 DFT 计算结果一致。即使在低水平的氧化或还原过程中,较强的 π 共轭作用也能实现全局电荷析出。这些发现为环状分子线的工程设计开辟了新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
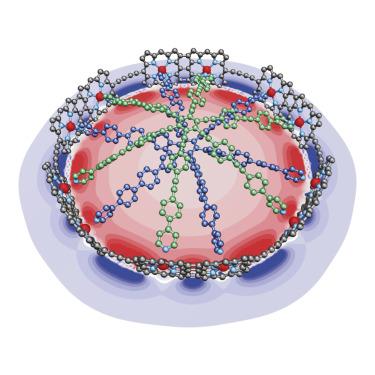
Charge delocalization and global aromaticity in a partially fused 12-porphyrin nanoring
Aromatic and antiaromatic ring currents can reveal global electronic delocalization around the circumference of π-conjugated macrocycles, although these phenomena are poorly understood in large rings. Here, we present the template-directed synthesis of a fully π-conjugated cyclic porphyrin 12-mer consisting of six β,meso,β-edge-fused porphyrin dimers connected by six butadiyne bridges. The lowest energy π-π∗ absorption band of this partially fused nanoring is shifted far into the NIR, confirming strong π-conjugation around the circumference of the macrocycle. Investigation of the oxidized and reduced nanoring-template complex by 1H and 19F NMR spectroscopy demonstrates the presence of coherent global (anti)aromatic ring currents, consistent with DFT calculations. The stronger π-conjugation enables global charge delocalization even at low levels of oxidation or reduction. These findings open new avenues for the engineering of cyclic molecular wires.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


