高熵材料:电化学水分离的潜在催化剂
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
作为新兴的电化学水分离催化剂,高熵材料(HEM)一直备受关注。与个位数催化剂相比,HEM 催化剂具有独特的性质,如有趣的鸡尾酒效应、广阔的设计空间、可定制的电子结构以及出色的熵稳定效果。本文全面概述了将 HEM 用作氢进化反应(HER)、氧进化反应(OER)和水分离催化剂的情况。本文讨论了 HEM 的设计策略,包括相结构调制、缺陷工程、电极配置工程和计算辅助工具,以开发具有高性能和稳定性的 HEM 催化剂。特别强调了密度泛函理论、高通量筛选技术和机器学习对发现和设计 HEM 催化剂的重要性。最后,展望了面临的挑战和即将面临的困难,并提出了应对这些挑战的相应策略,以推动 HEM 催化剂在电化学水分离领域的发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
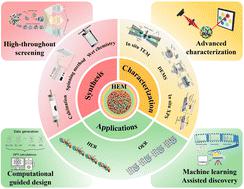
High entropy materials: potential catalysts for electrochemical water splitting
High entropy materials (HEM) have been attracting much attention as emerging catalysts for electrochemical water splitting. HEM catalysts have unique properties such as an interesting cocktail effect, broad design space, customizable electronic structure, and excellent entropy stabilization effect compared with single-digit catalysts. This paper provides a comprehensive overview of the use of HEM as a catalyst for hydrogen evolution reaction (HER), oxygen evolution reaction (OER), and water splitting. HEM design strategies including phase structure modulation, defect engineering, electrode configuration engineering, and computational aids are discussed to develop HEM catalysts with high performance and stability. In particular, the importance of density functional theory, high-throughput screening techniques, and machine learning for the discovery and design of HEM catalysts is emphasized. Finally, the challenges and imminent difficulties are prospected, and the corresponding strategies to deal with these challenges are put forward to promote the development of HEM catalysts in the field of electrochemical water splitting.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


