通过表面纳米形貌工程调节免疫细胞反应
IF 11.1
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
树突状细胞(DC)通过检测炎症信号、异常细胞或病原体,成为免疫反应的核心调节器。树突状细胞介导的免疫监视需要形态变化,以适应外部环境的物理和生化线索。这些变化由与表面受体相连的动态肌动蛋白细胞骨架-膜界面辅助,而表面受体将触发信号级联。近年来,合成免疫环境的发展使人们得以研究外部环境对免疫细胞反应的影响。在这一方向上,功能地形特征的生物工程应能确定膜形态如何调节直流电中的特定细胞功能。本文报告了通过软纳米压印光刻技术对一维纳米结构的二氧化硅表面进行工程化处理,以操纵体外人类 DCs 的膜形态。超分辨显微镜和活细胞成像研究表明,垂直柱状拓扑结构促进了DCs中富含黏性肌动蛋白结构的图案化和稳定化。此外,垂直拓扑结构还能刺激先天性免疫受体的空间组织,并调节细胞膜上由 Syk 和 ERK 介导的信号通路。总之,工程二氧化硅表面形貌可以通过施加局部质膜纳米变形来调节体外人类免疫细胞的细胞反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
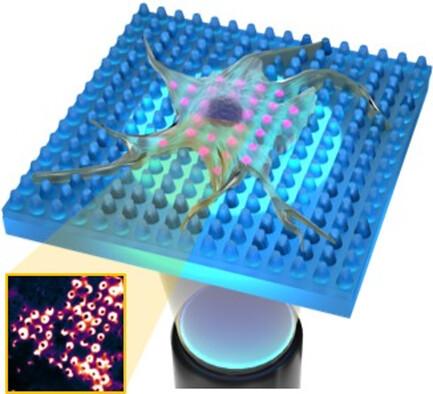
Tuning the Immune Cell Response through Surface Nanotopography Engineering
Dendritic cells (DCs) are central regulators of the immune response by detecting inflammatory signals, aberrant cells, or pathogens. DC-mediated immune surveillance requires morphology changes to adapt to the physical and biochemical cues of the external environment. These changes are assisted by a dynamic actin cytoskeleton–membrane interface connected to surface receptors that will trigger signaling cascades. In recent years, the development of synthetic immune environments has allowed to investigate the impact of the external environment in the immune cell response. In this direction, the bioengineering of functional topographical features should make it possible to establish how membrane morphology modulates specific cellular functions in DCs. Herein, the engineering of one-dimensional nanostructured SiO2 surfaces by soft-nanoimprint lithography to manipulate the membrane morphology of ex vivo human DCs is reported. Super-resolution microscopy and live-cell imaging studies show that vertical pillar topographies promote the patterning and stabilization of adhesive actin-enriched structures in DCs. Furthermore, vertical topographies stimulate the spatial organization of innate immune receptors and regulate the Syk- and ERK-mediated signaling pathways across the cell membrane. In conclusion, engineered SiO2 surface topographies can modulate the cellular response of ex vivo human immune cells by imposing local plasma membrane nano-deformations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
14.00
自引率
2.40%
发文量
0
期刊介绍:
Small Science is a premium multidisciplinary open access journal dedicated to publishing impactful research from all areas of nanoscience and nanotechnology. It features interdisciplinary original research and focused review articles on relevant topics. The journal covers design, characterization, mechanism, technology, and application of micro-/nanoscale structures and systems in various fields including physics, chemistry, materials science, engineering, environmental science, life science, biology, and medicine. It welcomes innovative interdisciplinary research and its readership includes professionals from academia and industry in fields such as chemistry, physics, materials science, biology, engineering, and environmental and analytical science. Small Science is indexed and abstracted in CAS, DOAJ, Clarivate Analytics, ProQuest Central, Publicly Available Content Database, Science Database, SCOPUS, and Web of Science.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


