通过明矾吸附调节咪唑喹啉类 TLR7/8 激动剂的佐剂潜力
IF 4
3区 医学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
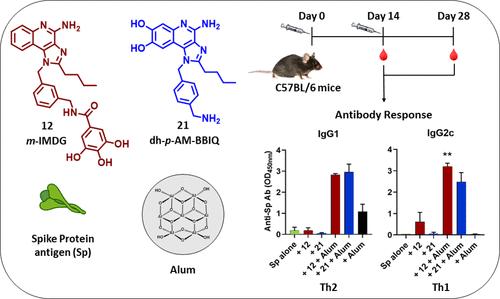
Toll样受体(TLR)-7/8激动剂是开发新一代疫苗佐剂的理想候选物质。TLR7/8 激动剂吸附在铝盐(明矾)上可进一步提高疫苗的免疫原性。对最具活性的双重 TLR7/8 激动剂 1-(3-(氨基甲基)苄基)-2-丁基-1H-咪唑并[4,5-c]喹啉-4-胺(m-AM-BBIQ,10)及其对位衍生物 p-AM-BBIQ (11)的佐剂性进行了评估、以及它们的没食子酸和原儿茶酸酰胺在基于重组蛋白的 COVID-19 疫苗平台中的应用,证实了 TLR7/8 激动剂中的沧海多酚功能对明矾吸附的重要性,从而产生平衡的 Th1/Th2 免疫反应。我们设计了一种新型 7,8- 二羟基-IMDQ 衍生物(dh-p-AM-BBIQ,21),其中在咪唑并[4,5-c]喹啉支架的喹啉环中引入了沧海多酚官能团。化合物 21 不仅保留了 TLR7 激动活性(EC50 = 3.72 μM),而且对明矾具有高吸附性,并能诱导免疫小鼠对 SARS-CoV-2 穗状病毒和乙型肝炎表面抗原产生强效抗体反应。由吸附在明矾上的化合物 21 组成的组合佐剂是一种很有希望进一步开发的人类和兽医疫苗佐剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Modulation of the Adjuvant Potential of Imidazoquinoline-Based TLR7/8 Agonists via Alum Adsorption
Toll-like receptor (TLR)-7/8 agonists are promising candidates for the development of new-generation vaccine adjuvants. Adsorption of TLR7/8 agonists on aluminum salts (alum) may further enhance vaccine immunogenicity. Evaluation of the adjuvanticity of the most active dual TLR7/8 agonists, 1-(3-(aminomethyl)benzyl)-2-butyl-1H-imidazo[4,5-c]quinolin-4-amine (m-AM-BBIQ, 10) and its para derivative p-AM-BBIQ (11), along with their gallic acid and protocatechuic acid amides in a recombinant-protein-based COVID-19 vaccine platform confirmed the importance of vic-polyphenolic functionality in TLR7/8 agonists for the alum adsorption, thereby resulting in a balanced Th1/Th2 immune response. A novel 7,8-dihydroxy-IMDQ derivative (dh-p-AM-BBIQ, 21) was designed wherein the vic-diphenolic functionality was introduced in the quinoline ring of the imidazo[4,5-c]quinoline scaffold. Compound 21 not only retained the TLR7 agonistic activity (EC50 = 3.72 μM) but also showed high adsorption to alum and induced a potent antibody response to SARS-CoV-2 spike protein and hepatitis B surface antigen immunized mice. The combination adjuvant comprising compound 21 adsorbed to alum represents a promising candidate for further development as a human and veterinary vaccine adjuvant.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
ACS Medicinal Chemistry Letters
CHEMISTRY, MEDICINAL-
CiteScore
7.30
自引率
2.40%
发文量
328
审稿时长
1 months
期刊介绍:
ACS Medicinal Chemistry Letters is interested in receiving manuscripts that discuss various aspects of medicinal chemistry. The journal will publish studies that pertain to a broad range of subject matter, including compound design and optimization, biological evaluation, drug delivery, imaging agents, and pharmacology of both small and large bioactive molecules. Specific areas include but are not limited to:
Identification, synthesis, and optimization of lead biologically active molecules and drugs (small molecules and biologics)
Biological characterization of new molecular entities in the context of drug discovery
Computational, cheminformatics, and structural studies for the identification or SAR analysis of bioactive molecules, ligands and their targets, etc.
Novel and improved methodologies, including radiation biochemistry, with broad application to medicinal chemistry
Discovery technologies for biologically active molecules from both synthetic and natural (plant and other) sources
Pharmacokinetic/pharmacodynamic studies that address mechanisms underlying drug disposition and response
Pharmacogenetic and pharmacogenomic studies used to enhance drug design and the translation of medicinal chemistry into the clinic
Mechanistic drug metabolism and regulation of metabolic enzyme gene expression
Chemistry patents relevant to the medicinal chemistry field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


