天然气成分在 2-(2-氨基乙氧基)乙醇 +1-Methylpyrrolidin-2-One/Water 混合溶剂中的溶解度
IF 2.1
3区 工程技术
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
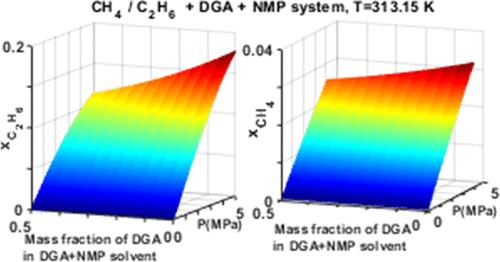
由于碳氢化合物是一种燃料来源,因此在气体增甜过程中溶剂中吸收碳氢化合物是不可取的。要解决这个问题并改进工艺设计和效率,就必须全面了解碳氢化合物的溶解特性。这些知识对于优化气体增甜工艺和提高整体生产效率至关重要。通过静态合成技术研究了甲烷和乙烷在胺质量分数为 0.3 和 0.5 的二甘醇胺、正甲基-2-吡咯烷酮和水的溶剂混合物中的溶解度。数据是在 313.15 K 和高达 2.185 MPa 的压力下等温测量的。实验数据使用 Soave-Redlich-Kwong 或 Peng-Robinson 状态方程建模。实验结果表明,用 n-甲基-2-吡咯烷酮代替二乙醇胺水溶液中的水会导致天然气主要成分的溶解度不理想地增加。不过,与 Purisol 工艺中使用的纯 n-甲基-2-吡咯烷酮相比,实验结果表明,在 Purisol 工艺中加入胺有可能增加二氧化碳的溶解度,降低甲烷和乙烷的溶解度。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Solubility of Natural Gas Components in Hybrid Solvents of 2-(2-aminoethoxy)ethanol +1-Methylpyrrolidin-2-One/Water
The absorption of hydrocarbons into the solvent during the gas-sweetening process is undesirable since this is a fuel source. To address this issue and improve the process design and efficiency, it is important to have a comprehensive understanding of the solubility characteristics of hydrocarbons. Such knowledge plays a crucial role in optimizing the gas-sweetening process and improving the overall production efficiency. The solubilities of methane and ethane in solvent mixtures of diglycolamine, n-methyl-2-pyrrolidone, and water with amine mass fractions of 0.3 and 0.5 were investigated via the static synthetic technique. The data were measured isothermally at 313.15 K with pressures of up to 2.185 MPa. The experimental data were modeled using either the Soave–Redlich–Kwong or Peng–Robinson equations of state. The experimental results indicate that substituting n-methyl-2-pyrrolidone for water in aqueous diglycolamine solvents results in an undesirable increase in the solubility of key natural gas components. However, compared to the use of pure n-methyl-2-pyrrolidone as in the Purisol process, the results show that incorporating amines into the Purisol process could potentially lead to increased carbon dioxide solubility and decreased methane and ethane solubility.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Chemical & Engineering Data
工程技术-工程:化工
CiteScore
5.20
自引率
19.20%
发文量
324
审稿时长
2.2 months
期刊介绍:
The Journal of Chemical & Engineering Data is a monthly journal devoted to the publication of data obtained from both experiment and computation, which are viewed as complementary. It is the only American Chemical Society journal primarily concerned with articles containing data on the phase behavior and the physical, thermodynamic, and transport properties of well-defined materials, including complex mixtures of known compositions. While environmental and biological samples are of interest, their compositions must be known and reproducible. As a result, adsorption on natural product materials does not generally fit within the scope of Journal of Chemical & Engineering Data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


