通过 B29 赖氨酸处的苯丙氨酸共轭合成高恒温胰岛素。
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
全球有数百万糖尿病患者需要每天注射胰岛素救命。胰岛素的热稳定性和纤维化带来了巨大的挑战,尤其是在世界上没有不间断冷藏设备的地区。在此,我们通过将胰岛素 B29 赖氨酸的ε-胺与乙酸、苯乙酸、丙氨酸和苯丙氨酸残基共轭,合成了四种人胰岛素类似物。其中,苯丙氨酸共轭胰岛素(称为 FHI)在高温(65 °C)、高盐胁迫(25 mM NaCl)和不同 pH 值(从高酸性 pH 1.6 到生理 pH 7.4)条件下最为稳定。它在体外、体内和体外抗纤维化的时间明显更长,具有持续的生物活性,并比其原生对应物显示出更长的稳定性。我们进一步揭示了关键的相互作用,如 FHI 中额外的芳香族 π-π 相互作用和氢键,而这些作用在原生胰岛素中是明显缺乏的。这些相互作用增强了 FHI 的结构稳定性,为解决与胰岛素热敏性相关的难题提供了一种有前景的解决方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。
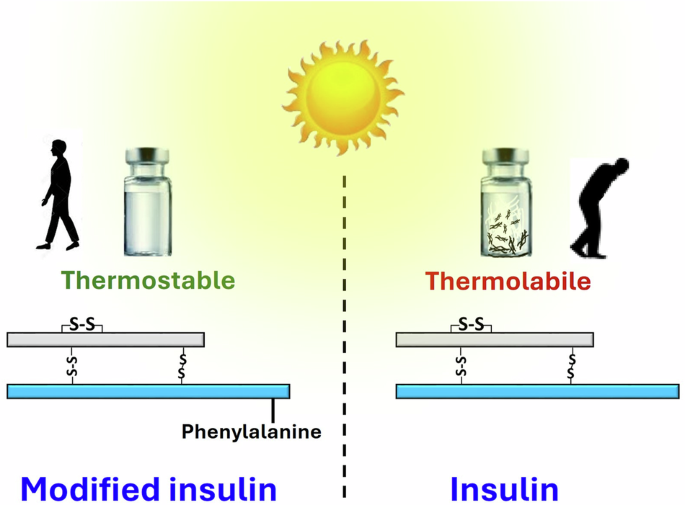
Synthesis of a highly thermostable insulin by phenylalanine conjugation at B29 Lysine
Globally, millions of diabetic patients require daily life-saving insulin injections. Insulin heat-lability and fibrillation pose significant challenges, especially in parts of the world without ready access to uninterrupted refrigeration. Here, we have synthesized four human insulin analogs by conjugating ε-amine of B29 lysine of insulin with acetic acid, phenylacetic acid, alanine, and phenylalanine residues. Of these, phenylalanine-conjugated insulin, termed FHI, was the most stable under high temperature (65 °C), elevated salt stress (25 mM NaCl), and varying pH levels (ranging from highly acidic pH 1.6 to physiological pH 7.4). It resists fibrillation for a significantly longer duration with sustained biological activity in in vitro, ex vivo, and in vivo and displays prolonged stability over its native counterpart. We further unravel the critical interactions, such as additional aromatic π-π interactions and hydrogen bonding in FHI, that are notably absent in native insulin. These interactions confer enhanced structural stability of FHI and offer a promising solution to the challenges associated with insulin heat sensitivity. Heat-lability and fibrillation of insulin pose significant challenges for insulin storage. Here, the authors report chemically modified analogs of insulin by functionalizing the ε-amine group of B29 Lys with phenylalanine, to improve insulin thermostability and resist fibrillation while maintaining robust in vivo activity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


