振荡自适应催化:立体选择性聚合内酯过程中由立体自校正调节的分子链内穿梭
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
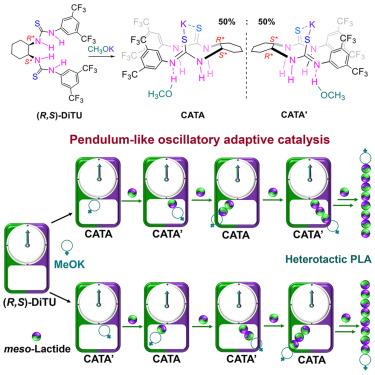
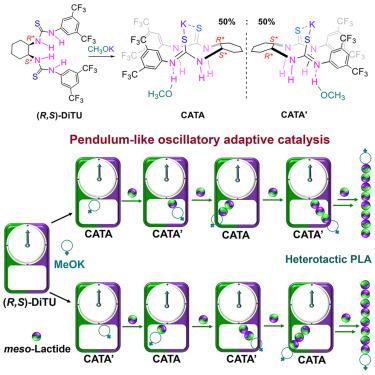
在金属介导的环酯立体选择性聚合反应中,外消旋催化剂利用其对映体发挥对映选择性作用,并通过分子间链交换实现多样化的聚合物立体结构。在这里,顺式 (R,S)- 二硫代脲和 MeOK 的组合实现了分子内链穿梭机制,从而克服了分子间聚合物交换的限制,并提供了多样化的聚合物立体结构。该体系对rac-内酯(rac-LA)和介-LA 的聚合具有非对映特异性,可分别生成高度同构聚乳酸(Pm ∼ 0.96)和异构聚乳酸(Pr ∼ 0.92)。机理研究揭示了一种 "振荡自适应催化"(OAC)现象,它是利用催化剂中的两个可切换手性中心实现对链端和进入单体的手性双重识别的关键。这种 OAC 通过多位点协同作用实现了手性识别(触发链的扩展)和立体化学自动校正(当单体不匹配时)之间的动态交换,从而在单个催化剂分子内诱导手性位点切换和聚合物链的分子内穿梭。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Oscillatory adaptive catalysis: Intramolecular chain shuttling regulated by stereo-autocorrection in stereoselective polymerization of lactide
In metal-mediated stereoselective polymerization of cyclic esters, racemic catalysts use their enantiomers for enantioselective roles and achieve diverse polymer stereomicrostructures through intermolecular chain exchange. Here, an intramolecular chain shuttling mechanism is achieved by the combination of cis (R,S)-dithiourea and MeOK to overcome limitations on intermolecular polymer exchange and also offer diverse polymer stereomicrostructures. This system exhibits diastereospecificity toward the polymerization of both rac-lactide (rac-LA) and meso-LA, producing highly isotactic PLA (Pm ∼ 0.96) and heterotactic PLA (Pr ∼ 0.92), respectively. Mechanistic studies reveal an “oscillatory adaptive catalysis” (OAC) phenomenon, which is key to achieving dual recognition of the chirality of both the chain end and incoming monomer by using the two switchable chiral centers in catalyst. Such OAC enables dynamic interchange between chiral recognition (that triggers chain propagation) and stereochemical autocorrection (when monomer mismatched) by multi-site cooperativity, which induces chiral-site switching and polymer-chain shuttling intramolecularly within a single catalyst molecule.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


