一种高效的 LOX 双噻唑抑制剂可重构胶原结构并增强三阴性乳腺癌的化疗反应
IF 6.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
赖氨酰氧化酶(LOX)在高度僵硬的侵袭性肿瘤中上调,与转移、耐药性和生存期恶化相关;然而,目前临床上还没有强效、安全、口服生物可用的小分子 LOX 抑制剂来治疗这些侵袭性脱落细胞实体瘤。在这里,我们通过对类药物分子的稳健筛选,结合基于细胞/重组蛋白的检测,发现了双噻唑衍生物作为强效的 LOX 抑制剂。结构-活性关系分析确定了一种强效先导化合物(LXG6403),它对 LOX 的特异性是 LOXL2 的 3.5 倍,同时不抑制 LOXL1,具有竞争性、时间和浓度依赖性的不可逆抑制模式。LXG6403 具有良好的药代动力学特性,能全面改变 ECM/胶原蛋白结构,降低肿瘤硬度。这使得药物渗透性更好,抑制了 FAK 信号转导,并诱导了 ROS/DNA 损伤、G1 停滞以及耐化疗三阴性乳腺癌(TNBC)细胞系、PDX 有机体和体内的细胞凋亡。总之,我们的双噻唑 LOX 抑制剂具有强效且可耐受的特点,能增强 TNBC(最致命的乳腺癌亚型)的化疗反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。

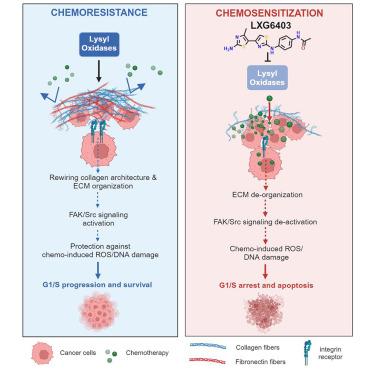
A highly potent bi-thiazole inhibitor of LOX rewires collagen architecture and enhances chemoresponse in triple-negative breast cancer
Lysyl oxidase (LOX) is upregulated in highly stiff aggressive tumors, correlating with metastasis, resistance, and worse survival; however, there are currently no potent, safe, and orally bioavailable small molecule LOX inhibitors to treat these aggressive desmoplastic solid tumors in clinics. Here we discovered bi-thiazole derivatives as potent LOX inhibitors by robust screening of drug-like molecules combined with cell/recombinant protein-based assays. Structure-activity relationship analysis identified a potent lead compound (LXG6403) with ∼3.5-fold specificity for LOX compared to LOXL2 while not inhibiting LOXL1 with a competitive, time- and concentration-dependent irreversible mode of inhibition. LXG6403 shows favorable pharmacokinetic properties, globally changes ECM/collagen architecture, and reduces tumor stiffness. This leads to better drug penetration, inhibits FAK signaling, and induces ROS/DNA damage, G1 arrest, and apoptosis in chemoresistant triple-negative breast cancer (TNBC) cell lines, PDX organoids, and in vivo. Overall, our potent and tolerable bi-thiazole LOX inhibitor enhances chemoresponse in TNBC, the deadliest breast cancer subtype.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


