将光生氮杂环丁烷醇开环作为合成氨基二氧戊环的一种策略
IF 2.2
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
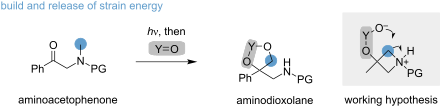
摘要α-氨基苯乙酮被认为是合成高取代二氧戊环的有前途的构筑基块。所提出的策略建立在建立和释放分子应变的基础上,并实现了一个甲基的形式转位。在光照射过程中,3-苯基氮杂环丁烷醇被合成为反应中间体,在加入缺电子的酮或硼酸后很容易发生开环反应。成功开发这种两步法的关键在于确定了一个苯甲基保护基团,该基团可协调光化学 Norrish–Yang 环化反应,并促进随后的开环反应。Chem.2024, 20, 1671–1676. doi:10.3762/bjoc.20.148本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ring opening of photogenerated azetidinols as a strategy for the synthesis of aminodioxolanes
Abstract
α-Aminoacetophenones are identified as promising building blocks for the synthesis of highly substituted dioxolanes. The presented strategy is founded on the build and release of molecular strain and achieves a formal transposition of a methyl group. During light irradiation, 3-phenylazetidinols are forged as reaction intermediates, which readily undergo ring opening upon the addition of electron-deficient ketones or boronic acids. Key to the successful development of this two-step process is the identification of a benzhydryl-protecting group, which orchestrates the photochemical Norrish–Yang cyclization and facilitates the subsequent ring opening.
Beilstein J. Org. Chem. 2024, 20, 1671–1676. doi:10.3762/bjoc.20.148
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
4.90
自引率
3.70%
发文量
167
审稿时长
1.4 months
期刊介绍:
The Beilstein Journal of Organic Chemistry is an international, peer-reviewed, Open Access journal. It provides a unique platform for rapid publication without any charges (free for author and reader) – Platinum Open Access. The content is freely accessible 365 days a year to any user worldwide. Articles are available online immediately upon publication and are publicly archived in all major repositories. In addition, it provides a platform for publishing thematic issues (theme-based collections of articles) on topical issues in organic chemistry.
The journal publishes high quality research and reviews in all areas of organic chemistry, including organic synthesis, organic reactions, natural product chemistry, structural investigations, supramolecular chemistry and chemical biology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


