肺炎链球菌 NADPH 氧化酶的结构和机理探究
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
烟酰胺腺嘌呤二核苷酸磷酸(NADPH)氧化酶(NOXs)通过介导活性氧的产生,在真核细胞的生理过程中发挥着重要作用。在细菌中发现了与 NOX 催化核心相距甚远的蛋白质,其中包括肺炎链球菌 NOX(SpNOX),由于其在洗涤剂胶束中的高活性和稳定性,SpNOX 被提议作为研究 NOX 的模型。我们在此展示了无底物和烟酰胺腺嘌呤二核苷酸(NADH)结合的 SpNOX 以及 NADPH 结合的野生型和 F397A SpNOX 在周转条件下的冷冻电镜结构。这些高分辨率结构提供了对电子转移途径的深入了解,并揭示了受 F397 位移调控的氢化物转移机制。我们进行了结构诱导突变和生化分析,解释了 NADPH 底物特异性的缺失,并提出了组成型活性背后的机制。我们的研究提出了 SpNOX 酶活性的结构基础,并揭示了其潜在的体内功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
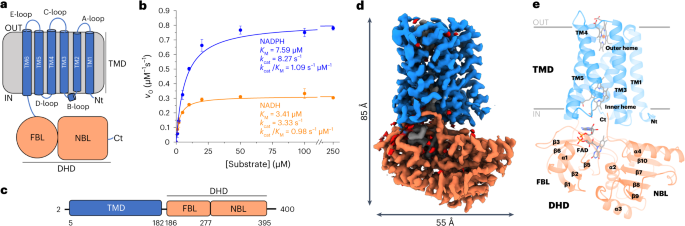
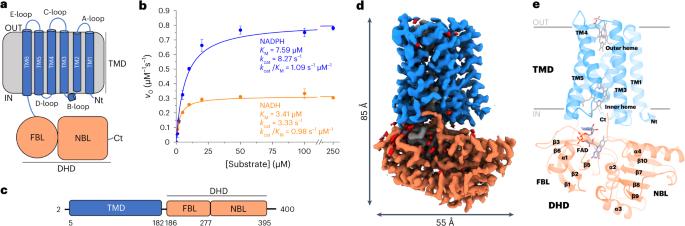
Structural and mechanistic insights into Streptococcus pneumoniae NADPH oxidase
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs) have a major role in the physiology of eukaryotic cells by mediating reactive oxygen species production. Evolutionarily distant proteins with the NOX catalytic core have been found in bacteria, including Streptococcus pneumoniae NOX (SpNOX), which is proposed as a model for studying NOXs because of its high activity and stability in detergent micelles. We present here cryo-electron microscopy structures of substrate-free and nicotinamide adenine dinucleotide (NADH)-bound SpNOX and of NADPH-bound wild-type and F397A SpNOX under turnover conditions. These high-resolution structures provide insights into the electron-transfer pathway and reveal a hydride-transfer mechanism regulated by the displacement of F397. We conducted structure-guided mutagenesis and biochemical analyses that explain the absence of substrate specificity toward NADPH and suggest the mechanism behind constitutive activity. Our study presents the structural basis underlying SpNOX enzymatic activity and sheds light on its potential in vivo function. Using cryo-electron microscopy, the authors obtained structures of Streptococcus pneumoniae nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in different states. Combined with site-directed mutagenesis and biochemical assays, the structures shed light on the activity and regulation of NADPH oxidases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


