核糖体终止复合体重塑释放因子 RF3 并排出 GDP。
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
翻译终止涉及释放因子 RF1、RF2 和 GTP 酶 RF3,后者从核糖体中回收 RF1 和 RF2。RF3 以 GDP 结合的形式从核糖体中解离,然后必须将 GDP 交换为 GTP。70S 核糖体终止复合体(70S-TC)可加速 RF3 的 GDP 交换,这表明 70S-TC 可充当 RF3 的鸟嘌呤核苷酸交换因子。在这里,我们利用低温电子显微镜阐明了大肠杆菌 70S-TC 催化 RF3 中 GDP 解离的机制。与 RF1 结合的非旋转核糖体重塑了 RF3,并诱导磷酸盐结合环中的肽翻转,从而有效地排出 GDP。GTP 的结合使 RF3 与 GTPase 中心对接,促进 RF1 与核糖体分离。这些结构再现了 RF3 在核糖体上的功能周期,并揭示了 70S-TC 异构拆除 RF3 磷酸盐结合槽的机制,这是以前被忽视的核糖体功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
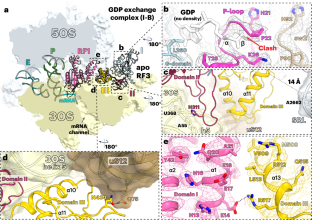

The ribosome termination complex remodels release factor RF3 and ejects GDP
Translation termination involves release factors RF1, RF2 and the GTPase RF3 that recycles RF1 and RF2 from the ribosome. RF3 dissociates from the ribosome in the GDP-bound form and must then exchange GDP for GTP. The 70S ribosome termination complex (70S-TC) accelerates GDP exchange in RF3, suggesting that the 70S-TC can function as the guanine nucleotide exchange factor for RF3. Here, we use cryogenic-electron microscopy to elucidate the mechanism of GDP dissociation from RF3 catalyzed by the Escherichia coli 70S-TC. The non-rotated ribosome bound to RF1 remodels RF3 and induces a peptide flip in the phosphate-binding loop, efficiently ejecting GDP. Binding of GTP allows RF3 to dock at the GTPase center, promoting the dissociation of RF1 from the ribosome. The structures recapitulate the functional cycle of RF3 on the ribosome and uncover the mechanism by which the 70S-TC allosterically dismantles the phosphate-binding groove in RF3, a previously overlooked function of the ribosome. Li et al. reveal the mechanism by which the ribosome termination complex catalyzes dissociation of GDP from release factor RF3 in Escherichia coli. The findings explain the guanine nucleotide exchange factor activity of the ribosome.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


