通过光诱导溶电子使药物中的炔烃发生氘化反应
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
人们已经认识到,将氘掺入药物分子会对药物疗效和安全性产生积极影响,从而改善药物动力学和/或毒性特征。由于烷烃对氢原子和电子转移过程具有很高的化学惰性,可见光通过氢同位素交换诱导芳香族 C(sp2)-H 键直接氘化的方法仍有待探索。在此,我们报告了一种光化学氘化方案,该方案只需一步就能将氘高效地掺入到烷烃中,并可容忍药物化合物中的多种官能团。机理研究证明,溶解电子是在苯酚型光催化剂的光照下产生的,并参与了氘的掺入。该方案被成功应用于通过光化学芳香族 H/D 交换在烷上对药物进行后期氘化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
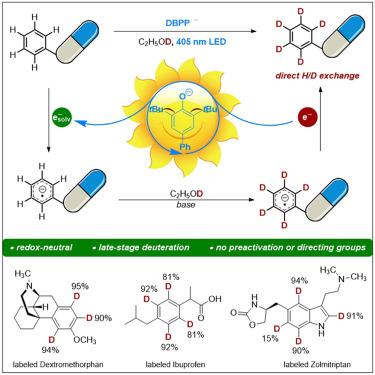
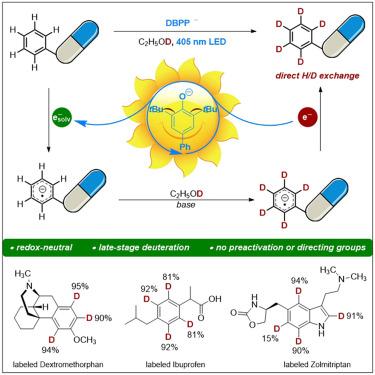
Deuteration of arenes in pharmaceuticals via photoinduced solvated electrons
Deuterium incorporation into pharmaceutical molecules has been recognized as having a positive impact on drug efficacy and safety, allowing improvements in pharmacokinetic and/or toxicity profiles. Due to the high chemical inertness of arenes toward the hydrogen atom and the electron transfer processes, the visible light-induced direct deuteration of aromatic C(sp2)–H bonds via hydrogen isotope exchange remains unexplored. Herein, we report a photochemical deuteration protocol for efficient incorporation of deuterium into arenes in a single step, tolerating manifold functionalities in pharmaceutical compounds. Mechanistic studies provided evidence that solvated electrons were generated by light illumination with a phenolate-type photocatalyst and were involved in deuterium incorporation. This protocol was successfully applied to the late-stage deuteration of pharmaceuticals by photochemical aromatic H/D exchange on arenes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


