蛋白质半合成揭示了 HECT E3 泛素连接酶机制的可塑性
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
赖氨酸泛素化由 E3 泛素连接酶催化,是调节正常和疾病状态下蛋白质稳定性和细胞信号的核心。我们对 E3 机制的认识还存在差距,在此,我们使用蛋白质半合成、化学救援、微尺度热泳和其他生化方法来剖析催化碱基/酸功能和构象相互转换在 HECT 域 E3 催化中的作用。我们证明,在 HECT E3 泛素连接酶反应中,利用末端侧链或骨架羧酸盐进行质子转移具有可塑性,酵母 Rsp5 同源物似乎是可能的进化中间体。我们还发现,HECT-domain 泛素共价中间体似乎能弹射出 E2 连接酶,促进催化周转。这些发现为了解蛋白质泛素化的发生提供了关键的机理见解,并为了解 E3 的功能和调控提供了一个框架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
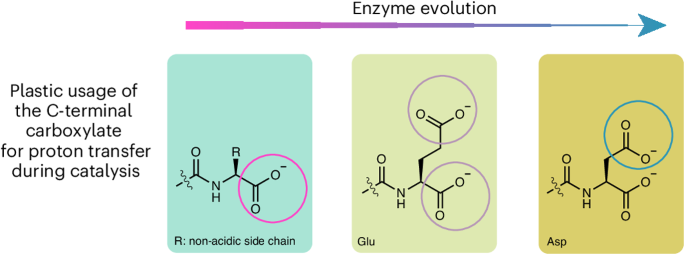
Protein semisynthesis reveals plasticity in HECT E3 ubiquitin ligase mechanisms
Lys ubiquitination is catalysed by E3 ubiquitin ligases and is central to the regulation of protein stability and cell signalling in normal and disease states. There are gaps in our understanding of E3 mechanisms, and here we use protein semisynthesis, chemical rescue, microscale thermophoresis and other biochemical approaches to dissect the role of catalytic base/acid function and conformational interconversion in HECT-domain E3 catalysis. We demonstrate that there is plasticity in the use of the terminal side chain or backbone carboxylate for proton transfer in HECT E3 ubiquitin ligase reactions, with yeast Rsp5 orthologues appearing to be possible evolutionary intermediates. We also show that the HECT-domain ubiquitin covalent intermediate appears to eject the E2 conjugating enzyme, promoting catalytic turnover. These findings provide key mechanistic insights into how protein ubiquitination occurs and provide a framework for understanding E3 functions and regulation. Lysine ubiquitination, catalysed by E3 ubiquitin ligases, is pivotal for regulating protein stability and cell signalling. Using protein semisynthesis, the roles of the C-terminal carboxylate and conformational interconversion in HECT-domain E3 catalysis are now characterized, revealing evolutionary plasticity in side chain versus backbone utilization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


