碰撞排列和分子旋转控制着对苯二酚单个构象与可迁移氖的化学电离
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
分子形状与其化学反应性之间的关系是化学的核心原理。然而,分子几何形状对反应性的影响可能是微妙的,并且是由几种相反的效应造成的。在这里,我们利用交叉分子束实验,分离了分子特定构象的各个旋转量子态,研究了对苯二酚与氖原子的化学电离反应。我们的研究表明,由几何长程力引起的反应伙伴碰撞诱导对齐会影响反应路径,但分子旋转会抵消这种影响。本研究揭示了复杂多原子体系的构象特异性立体动力学,并展示了先进的分子控制技术揭示这些效应的能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
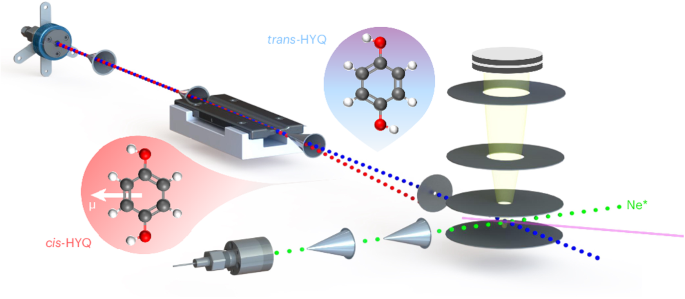
Collisional alignment and molecular rotation control the chemi-ionization of individual conformers of hydroquinone with metastable neon
The relationship between the shape of a molecule and its chemical reactivity is a central tenet in chemistry. However, the influence of the molecular geometry on reactivity can be subtle and result from several opposing effects. Here, using a crossed-molecular-beam experiment in which individual rotational quantum states of specific conformers of a molecule are separated, we study the chemi-ionization reaction of hydroquinone with metastable neon atoms. We show that collision-induced alignment of the reaction partners caused by geometry-dependent long-range forces influences reaction pathways, which is, however, countered by molecular rotation. The present work provides insights into the conformation-specific stereodynamics of complex polyatomic systems and illustrates the capability of advanced molecule-control techniques to unravel these effects. Molecular geometry can influence chemical reactivity through several opposing effects. By selecting individual conformers of hydroquinone in the chemi-ionization reaction with metastable neon, it is now shown that reaction pathways can be governed by molecular alignment due to geometry-dependent forces that are, however, countered by molecular rotation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


