进化的人工自由基环化酶可通过 H 原子转移途径构建双环萜类支架
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
天然萜类环化酶通过阳离子机制生成复杂的萜类结构,但对其他自由基环化途径的探索还不够。金属催化的 H 原子转移反应(M-HAT)为烯烃的氢官能化提供了一种极具吸引力的方法,从而提供了获得类萜结构的途径。人工金属酶为将 M-HAT 反应性引入蛋白质支架提供了一种前景广阔的策略。在此,我们报告了通过将生物素化的[Co(希夫碱)]辅助因子锚定在工程化的嵌合链霉亲和素中,我们在工程化人工自由基环化酶(ARCase)方面所做的努力。经过两轮定向进化后,一个双突变体催化了自由基环化,产生了具有顺式-5-6-融合环结构的双环产物,对映体过量率高达 97%。晶体学研究证实了组氨酸与 Co 辅助因子的连接。时程实验揭示了 ARCase 催化的级联反应,它将自由基环化与共轭还原结合在一起。ARCase 对二烯酮底物的变化表现出耐受性,突显了其获取萜类支架的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
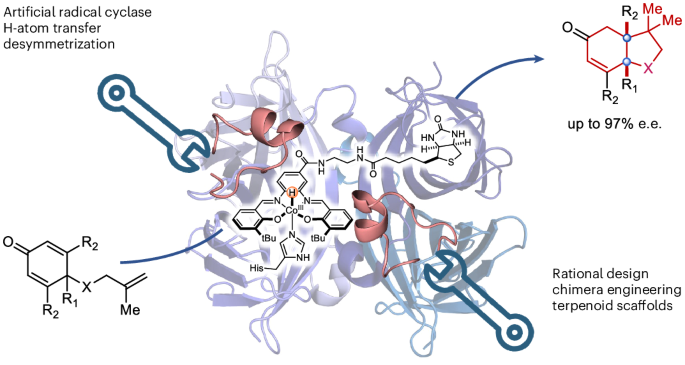
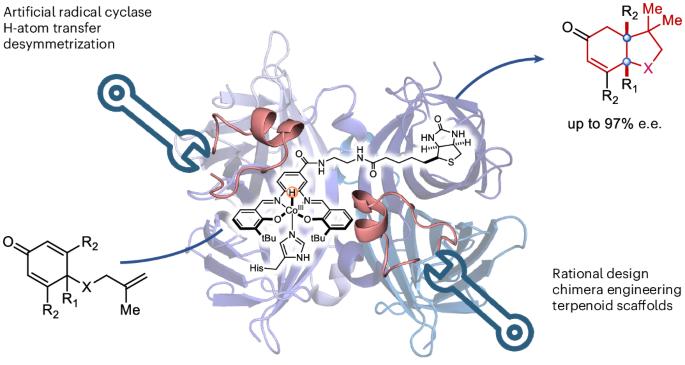
An evolved artificial radical cyclase enables the construction of bicyclic terpenoid scaffolds via an H-atom transfer pathway
While natural terpenoid cyclases generate complex terpenoid structures via cationic mechanisms, alternative radical cyclization pathways are underexplored. The metal-catalysed H-atom transfer reaction (M-HAT) offers an attractive means for hydrofunctionalizing olefins, providing access to terpenoid-like structures. Artificial metalloenzymes offer a promising strategy for introducing M-HAT reactivity into a protein scaffold. Here we report our efforts towards engineering an artificial radical cyclase (ARCase), resulting from anchoring a biotinylated [Co(Schiff-base)] cofactor within an engineered chimeric streptavidin. After two rounds of directed evolution, a double mutant catalyses a radical cyclization to afford bicyclic products with a cis-5-6-fused ring structure and up to 97% enantiomeric excess. The involvement of a histidine ligation to the Co cofactor is confirmed by crystallography. A time course experiment reveals a cascade reaction catalysed by the ARCase, combining a radical cyclization with a conjugate reduction. The ARCase exhibits tolerance towards variations in the dienone substrate, highlighting its potential to access terpenoid scaffolds. Although natural terpenoid cyclases generate polycyclic structures through cationic intermediates, alternative radical cyclization pathways are underexplored. Now an artificial radical cyclase has been prepared by anchoring a biotinylated cobalt Schiff-base complex within a chimeric streptavidin scaffold. Chemogenetic optimization of the catalytic performance affords enantioenriched terpenoids via a metal-catalysed H-atom transfer mechanism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


