利用可回收离子液体催化剂对氢硅烷进行电化学氧化脱氢以生成硅基:一种构建 Si-O/Si-Si 键的高效方法
IF 9.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
通过氢硅烷与 O 型亲核物(如酚、萘酚、醇和 H2O)的电化学氧化脱氢反应或氢硅烷自缩合反应,开发出了一种构建 Si-O/Si-Si 键的高效、可持续方法。该方案采用高导电性和可回收的离子液体作为催化剂,因此无需外加电解质和氢原子转移(HAT)剂。离子液体可轻松回收并重复使用至少八个周期,且性能始终如一。值得注意的是,这种电化学方法具有广泛的底物范围和较高的官能团兼容性(66 个实例,收率高达 96%)。初步的机理研究表明,硅自由基是通过溴自由基和硅烷之间的氢原子转移过程产生的,KIE 实验证明,Si-H 键裂解是该反应的决定性步骤。本文章由计算机程序翻译,如有差异,请以英文原文为准。
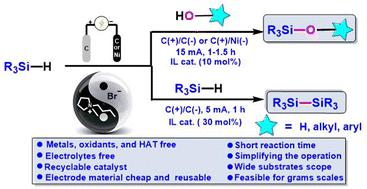
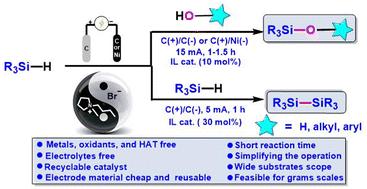
Electrochemical oxidative dehydrogenation of hydrosilanes to generate silyl radicals: an efficient method for the construction of Si–O/Si–Si bonds utilizing a recyclable ionic liquid catalyst†
A highly efficient and sustainable approach was developed for the construction of Si–O/Si–Si bonds, through the electrochemical oxidative dehydrogenation of hydrosilanes with O-nucleophiles (e.g. phenols, naphthols, alcohols, and H2O) or hydrosilane self-condensation. The protocol employs a highly electrically conductive and recyclable ionic liquid as a catalyst, thus eliminating the need for external electrolytes and hydrogen atom transfer (HAT) agents. The ionic liquid could be easily recovered and reused for at least eight cycles with consistent performance. Notably, this electrochemical method exhibits a broad substrate scope and high functional-group compatibility (66 examples, up to 96% yield). Initial mechanistic studies show that silicon radicals are generated via the process of hydrogen atom transfer between bromine radicals and silanes, and KIE experiments demonstrate that Si–H bond cleavage is the rate-determining step of the reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


