在水氧化还原液流电池中使用曼尼希反应制备氨基蒽醌
IF 6.2
Q2 ENERGY & FUELS
引用次数: 0
摘要
在 pH 值为 14 的水氧化还原液流电池的负电位电解质(电解液)中引入了一种从茜素中提取的水溶性蒽醌 3HAAQ,作为氧化还原活性材料。3HAAQ 的合成是通过曼尼希反应进行的,该反应大大提高了新化合物的溶解度,这是将其用于液流氧化还原电池的一个重要因素。这种液流电池与铁氰化钾/铁氰化铁正极电解质配对,开路电压为 1.24 V,在 40 mA cm-2 电流密度下可保持近 80% 的理论容量。本文章由计算机程序翻译,如有差异,请以英文原文为准。
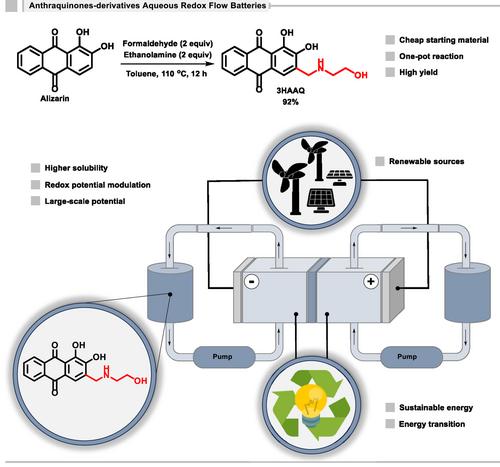
The Use of the Mannich Reaction toward Amino-Based Anthraquinone Applied in Aqueous Redox Flow Battery
A water-soluble anthraquinone derived from alizarin, 3HAAQ, is introduced as the redox-active material in a negative potential electrolyte (anolyte) for aqueous redox flow batteries operating at pH 14. The synthesis of 3HAAQ is carried out using the Mannich reaction, which significantly improves the solubility of the new compound, an important factor for its use in RFB. Pairing with potassium ferri/ferrocyanide positive electrolyte, this flow battery exhibits an open-circuit voltage of 1.24 V and maintains nearly 80% of the theoretical capacity at 40 mA cm−2 current density.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.20
自引率
3.40%
发文量
0
期刊介绍:
Advanced Energy and Sustainability Research is an open access academic journal that focuses on publishing high-quality peer-reviewed research articles in the areas of energy harvesting, conversion, storage, distribution, applications, ecology, climate change, water and environmental sciences, and related societal impacts. The journal provides readers with free access to influential scientific research that has undergone rigorous peer review, a common feature of all journals in the Advanced series. In addition to original research articles, the journal publishes opinion, editorial and review articles designed to meet the needs of a broad readership interested in energy and sustainability science and related fields.
In addition, Advanced Energy and Sustainability Research is indexed in several abstracting and indexing services, including:
CAS: Chemical Abstracts Service (ACS)
Directory of Open Access Journals (DOAJ)
Emerging Sources Citation Index (Clarivate Analytics)
INSPEC (IET)
Web of Science (Clarivate Analytics).

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


