浅俯冲带硅质流体介导的富氧碳酸盐反应
IF 8.9
1区 地球科学
Q1 GEOSCIENCES, MULTIDISCIPLINARY
引用次数: 0
摘要
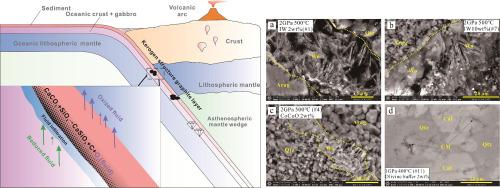
沉积物是俯冲带的主要碳汇之一,CaCO3和SiO2是沉积物的主要成分。它们的化学稳定性对地幔中碳的形式起着重要作用。本文报道了在0.8 ~ 2.0 GPa、400 ~ 500℃、浅俯冲带氧化还原缓冲条件下,CaCO3与SiO2在水合沉积物中的反应。结果表明,在coco和IW缓冲条件下,CaCO3 + SiO2 = CaSiO3 + C + O2(流体)反应生成硅灰石(CaSiO3)和碳质物质(CM)。此外,硅灰石是由溶解结晶过程形成的,这一过程可能受氧逸度的影响较大,导致其结晶习惯不同(Yui, 1966, Schott et al., 2012)。无水实验表明,在本研究的压力和温度(P-T)范围内,只有H2O存在时反应才会进行。文石结构的CaCO3比方解石结构的CaCO3反应更快。在1.0 GPa、400℃条件下用天然橄榄石缓冲的实验证明,上述反应在浅层俯冲带蛇纹石化过程中是可能发生的。更重要的是,纳米级CM可以在相对还原的条件下生成,表现出干酪根的拉曼特征。这些结果为了解碳在地球内部分布的深度提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Oxygen fugacity-mediated carbonate reactions with siliceous fluids in shallow subduction zones
Sediments are one of the main carbon sinks in subduction zones, with CaCO3 and SiO2 being the main components in sediments. Their chemical stability plays a significant role in the form of carbon in the Earth’s mantle. Here we report the reactions of CaCO3 with SiO2 in hydrated sediments at 0.8–2.0 GPa, 400–500 ℃ and redox-buffered conditions relevant to shallow subduction zones. Our results show that the reaction CaCO3 + SiO2 = CaSiO3 + C + O2(fluid) occurs under CoCoO and IW buffered conditions to generate wollastonite (CaSiO3) and carbonaceous material (CM). Moreover, wollastonite is formed by the dissolution-crystallization process, which may be significantly affected by oxygen fugacity, leading to distinct crystallization habits (Yui, 1966, Schott et al., 2012). Anhydrous experiments indicate that the reaction proceeds only in the presence of H2O within the pressure and temperature (P-T) range of this study. The reaction occurs more rapidly with aragonite-structured than calcite-structured CaCO3. Further, the experiment buffered with natural olivine at 1.0 GPa and 400 ℃ proves that the above reaction can occur during serpentinization processes in shallow subduction zones. More importantly, nanoscale CM may be generated under relatively reducing conditions, exhibiting Raman characteristics of kerogen. These results provide new insights into how deep carbon is distributed in the Earth’s interior.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Geoscience frontiers
Earth and Planetary Sciences-General Earth and Planetary Sciences
CiteScore
17.80
自引率
3.40%
发文量
147
审稿时长
35 days
期刊介绍:
Geoscience Frontiers (GSF) is the Journal of China University of Geosciences (Beijing) and Peking University. It publishes peer-reviewed research articles and reviews in interdisciplinary fields of Earth and Planetary Sciences. GSF covers various research areas including petrology and geochemistry, lithospheric architecture and mantle dynamics, global tectonics, economic geology and fuel exploration, geophysics, stratigraphy and paleontology, environmental and engineering geology, astrogeology, and the nexus of resources-energy-emissions-climate under Sustainable Development Goals. The journal aims to bridge innovative, provocative, and challenging concepts and models in these fields, providing insights on correlations and evolution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


