作为碘化试剂的碘(I)核苷酸络合物。
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
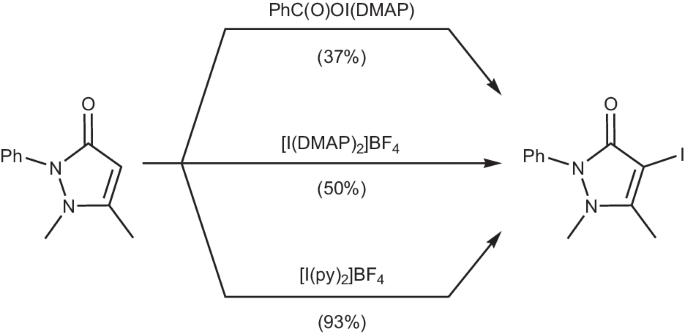
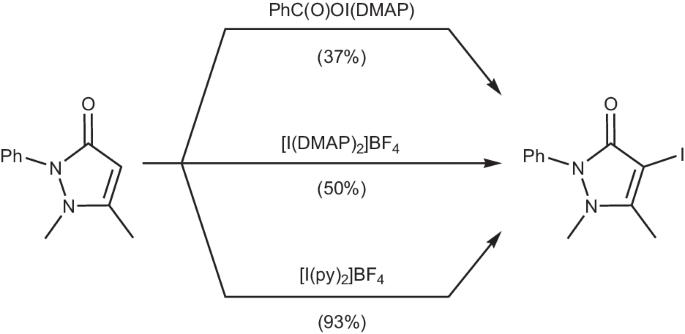
卤素(I)络合物被广泛用作卤化试剂,传统上以同色稳定的路易斯碱为特征,不过最近碘(I)羧酸盐化学的复兴提供了可分离的异色碘(I)络合物实例。本研究报告介绍了由路易斯碱(L)(Ph2P(O)O─I─L)稳定的碘(I)吡啶羧酸盐络合物,该络合物是通过阳离子交换从银(I)前体 (Ph2P(O)OAg)n 合成的。这些配合物在溶液(1H、1H-15N HMBC、31P)和固态下都有特征,并通过 DFT 研究进行了计算补充。有趣的是,这些碘(I)pnictogenates 具有不同的稳定性,与羰基次碘化物相比,它们在碘化安替比林时的反应活性与同名的 Barluenga 试剂相当。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Iodine(I) pnictogenate complexes as Iodination reagents
Halogen(I) complexes are widely used as halogenation reagents and traditionally feature homoleptic stabilising Lewis bases, though the recent revitalisation of iodine(I) carboxylate chemistry has provided isolable examples of heteroleptic iodine(I) complexes. This work reports iodine(I) pnictogenate complexes stabilised by a Lewis base (L), Ph2P(O)O─I─L, synthesised via cation exchange from the silver(I) precursor, (Ph2P(O)OAg)n. The complexes were characterised in both solution (1H, 1H-15N HMBC, 31P) and the solid state, and supplemented computationally by DFT studies. Interestingly, these iodine(I) pnictogenates demonstrate a range of stabilities, and have been found to excel as iodination reagents in comparison to carbonyl hypoiodites, with comparable reactivity to the eponymous Barluenga’s reagent in the iodination of antipyrine. Iodine(I) carboxylates have been explored as iodination reagents, but the role of the carbonyl group in promoting such reactivity remains poorly understood. Here, the authors prepare iodine(I) pnictogenates and find that they excel as iodination reagents in comparison to iodine(I) carboxylates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


