MASH 相关代偿性肝硬化临床试验路线图
IF 45.9
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
尽管代谢功能障碍相关性脂肪性肝炎(MASH)正迅速成为全球肝硬化的主要病因,但治疗方案却很有限,而且与早期疾病阶段的临床试验相比,针对 MASH 相关代偿性肝硬化的临床试验数量很少。此外,MASH 肝硬化临床试验的设计也面临着一系列挑战,包括对自然史的理解和概念化、监管考虑、纳入标准、招募、终点和试验持续时间等。2023 年 4 月,在西班牙巴塞罗那 Vall d'Hebron 大学医院举办了首届关于 MASH 相关代偿性肝硬化临床试验的最新进展和未来方向的国际研讨会,来自学术界、监管机构和产业界的临床试验国际专家参加了此次研讨会,他们在 MASH、肝硬化、门静脉高压症和监管事务方面拥有丰富的专业知识。所提交的路线图总结了研讨会的重要内容,涉及 MASH 相关代偿性肝硬化临床试验的现状、监管要求和终点,探讨了可供选择的研究设计,并强调了即将开展的 MASH 肝硬化研究应考虑的挑战。本文章由计算机程序翻译,如有差异,请以英文原文为准。
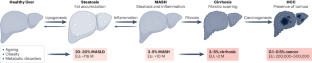
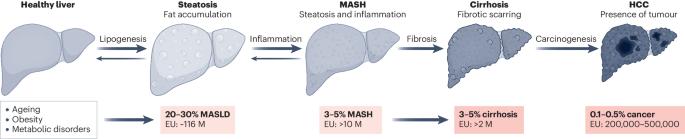
A roadmap for clinical trials in MASH-related compensated cirrhosis
Although metabolic dysfunction-associated steatohepatitis (MASH) is rapidly becoming a leading cause of cirrhosis worldwide, therapeutic options are limited and the number of clinical trials in MASH-related compensated cirrhosis is low as compared to those conducted in earlier disease stages. Moreover, designing clinical trials in MASH cirrhosis presents a series of challenges regarding the understanding and conceptualization of the natural history, regulatory considerations, inclusion criteria, recruitment, end points and trial duration, among others. The first international workshop on the state of the art and future direction of clinical trials in MASH-related compensated cirrhosis was held in April 2023 at Vall d’Hebron University Hospital in Barcelona (Spain) and was attended by a group of international experts on clinical trials from academia, regulatory agencies and industry, encompassing expertise in MASH, cirrhosis, portal hypertension, and regulatory affairs. The presented Roadmap summarizes important content of the workshop on current status, regulatory requirements and end points in MASH-related compensated cirrhosis clinical trials, exploring alternative study designs and highlighting the challenges that should be considered for upcoming studies on MASH cirrhosis. Metabolic dysfunction-associated steatohepatitis (MASH), a primary cause of chronic liver disease (CLD), often leads to advanced CLD stages such as cirrhosis. This Roadmap summarizes the current landscape and challenges of MASH-related compensated cirrhosis clinical trials and explores a way forward for future studies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
52.30
自引率
0.60%
发文量
147
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Gastroenterology & Hepatology aims to serve as the leading resource for Reviews and commentaries within the scientific and medical communities it caters to. The journal strives to maintain authority, accessibility, and clarity in its published articles, which are complemented by easily understandable figures, tables, and other display items. Dedicated to providing exceptional service to authors, referees, and readers, the editorial team works diligently to maximize the usefulness and impact of each publication.
The journal encompasses a wide range of content types, including Research Highlights, News & Views, Comments, Reviews, Perspectives, and Consensus Statements, all pertinent to gastroenterologists and hepatologists. With its broad scope, Nature Reviews Gastroenterology & Hepatology ensures that its articles reach a diverse audience, aiming for the widest possible dissemination of valuable information.
Nature Reviews Gastroenterology & Hepatology is part of the Nature Reviews portfolio of journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


